Schizophrenia-associated alterations in fecal mycobiota and systemic immune dysfunction: a cohort study of elderly Chinese patients
- PMID: 40761785
- PMCID: PMC12318963
- DOI: 10.3389/fimmu.2025.1607739
Schizophrenia-associated alterations in fecal mycobiota and systemic immune dysfunction: a cohort study of elderly Chinese patients
Abstract
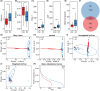
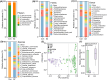
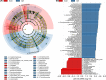
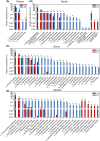
Schizophrenia (SZ) is a severe psychiatric disorder with a complex etiology involving both genetic and environmental factors. Emerging evidence highlights the role of gut microbiome dysbiosis in SZ, yet the fungal component (mycobiota) remains largely unexplored. This study aimed to evaluate the gut mycobiota using internal transcribed spacer 1 (ITS1) amplicon sequencing and assess host immune responses via multiplex immunoassays in 87 elderly SZ patients and 64 age- and gender-matched healthy controls (HCs). We observed significant increases in fungal α-diversity and richness, along with altered β-diversity in SZ patients. Specifically, there was an elevated Basidiomycota/Ascomycota ratio, with enrichment of Candida, Aspergillus, and Saccharomyces, coupled with a depletion of Purpureocillium. Enterotype analysis revealed a shift from Purpureocillium-dominant (E1) to Candida-dominant (E2) communities in SZ. Notably, key fungal species, such as S. cerevisiae and P. lilacinum, were correlated with systemic immune dysfunction. Our receiver operating characteristic (ROC) analysis indicated that these fungal species could effectively distinguish SZ patients from HCs, suggesting their potential as non-invasive biomarkers for SZ diagnosis. In conclusion, this study demonstrates significant alterations in the gut mycobiota and immune dysfunction in elderly SZ patients, suggesting that mycobiota dysbiosis may contribute to SZ pathogenesis through immune modulation, offering new avenues for potential biomarkers and therapeutic interventions.
Keywords: Candida; Purpureocillium; gut mycobiota; immune dysfunction; schizophrenia.
Copyright © 2025 Ling, Cheng, Liu, Xu, Wu, Shao, Zhu, Ding, Song, Zhao and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

