Anti-CD19 CAR-T cell therapy in relapsed/refractory t(8;21) acute myeloid leukemia with aberrant CD19 expression
- PMID: 40761794
- PMCID: PMC12318730
- DOI: 10.3389/fimmu.2025.1617589
Anti-CD19 CAR-T cell therapy in relapsed/refractory t(8;21) acute myeloid leukemia with aberrant CD19 expression
Abstract
Background: T (8; 21) acute myeloid leukemia (AML) is a special type of acute leukemia, and exhibits a heterogeneous prognosis, with a long-term relapse rate of about 40%. Once t(8; 21) AML patients experience relapse, they have an extremely poor prognosis, with a 5-year overall survival rate of less than 15%. Therefore, it is crucial to develop effective strategies to improve the prognosis of relapsed/refractory (R/R) t(8; 21) AML. CD19 is a specific B-cell surface marker, but it is aberrantly expressed in 50-80 % of t(8; 21) AML patients. CAR-T cells targeting aberrant cell-surface antigens could induce the depletion of tumor cells without the destruction of hematopoiesis. Therefore, CD19 might be a promising target for CAR-T cell therapy in R/R t(8; 21) AML with aberrant CD19 expression. The present study is aimed to explore the efficacy and safety of CD19 CAR-T cell therapy in R/R t(8;21) AML with aberrant CD19 expression.
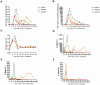
Methods: In the present study, 3 R/R t(8;21) AML patients with aberrant CD19 expression were enrolled. After lymphodepleting chemotherapy, 3 patients received autologous CAR-T cell infusion at a dose of 1.0 × 10^6 cells/kg, 2.0 × 10^6 cells/kg, and 2.0 × 10^6 cells/kg, respectively.
Results: They all achieved CD19 negativity approximately half a month after CD19 CAR-T cell infusion. These indicate CD19 CAR-T cell therapy is effective in R/R t(8;21) AML with aberrant CD19 expression. However, patient 1 and patient 2 rapidly relapsed within 3 months after CD19 CAR-T cell therapy. Subsequently, patient 1 received allogeneic hematopoietic stem cell transplantation (allo-HSCT). Fortunately, patient 1 achieved mCR 2 months after allo-HSCT.
Conclusion: Considering the short-term remission of CD19 CAR-T cell therapy in R/R t(8;21) AML, allo-HSCT might be performed as soon as possible to consolidate the efficacy of CAR-T cell therapy and reduce the risk of relapse.
Keywords: CD19 CAR-T cell therapy; aberrant CD19 expression; hematological remission; molecular remission; t(8; 21) acute myeloid leukemia.
Copyright © 2025 Zhang, Wang, Qiao, Wang, Wang, Liu, Qin, Chen, Huang, Zheng, Peng, Mei, Wang, Yu, Li and Yu.
Conflict of interest statement
Authors CY and YL were employed by the company Shenzhen Haoshi Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. (2010) 116:354–65. doi: 10.1182/blood-2009-11-254441, PMID: - DOI - PubMed
-
- Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. (2016) 51:1431–8. doi: 10.1038/bmt.2016.167, PMID: - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

