MetE: a promising protective antigen for tuberculosis vaccine development
- PMID: 40761798
- PMCID: PMC12319054
- DOI: 10.3389/fimmu.2025.1593263
MetE: a promising protective antigen for tuberculosis vaccine development
Abstract
Introduction: Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), remains a significant global health concern. The existing vaccine, Bacillus Calmette-Guérin (BCG), provides inconsistent protection, highlighting the pressing need for a more effective vaccine. We aimed to identify novel MTB antigens and assess their protective efficacy as TB vaccine candidates.
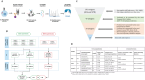
Methods: Using immunopeptidomics, we identified 64 and 80 unique mycobacterial antigens derived from BCG and MTB, respectively. We prioritised antigens based on HLA allele coverage through an immunoinformatics approach.
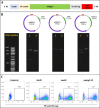
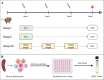
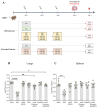
Results: The candidates, hisD, metE, and mmpL12, delivered as DNA vaccines, were evaluated for efficacy in mice using the ex vivo Mycobacterial Growth Inhibition Assay (MGIA) and metE was identified as a promising candidate. In vivo murine MTB challenge experiments confirmed the protective efficacy conferred by metE when formulated as recombinant protein with AS01™ or AddaS03™ adjuvants, compared to the naïve group. The immunogenic profiles of metE formulated in the two different adjuvants differed, with metE-AS01™ inducing antigen-specific IFN-γ, TNF-α, IL-2, IL-17, IgG1 and IgG2a-c, while metE-AddaS03™ induced TNF-α, IL-2, IL-17, IL-4, IgM, IgG1, IgG2b.
Conclusion: Our findings highlight metE as a promising protective antigen for future TB vaccine development.
Keywords: HLA/MHC; antigen discovery; immunoinformatics; immunopeptidomics; mass spectrometry; mycobacterium tuberculosis; tuberculosis; vaccines.
Copyright © 2025 Almujri, Stylianou, Nicastri, Satti, Korompis, Li, De Voss, Polo Peralta Alvarez, Tanner, Bettencourt, Ternette and McShane.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Global tuberculosis report 2024. Geneva: World Health Organization; (2024). Available online at: https://www.who.int/publications/i/item/9789240101531.
-
- Rieder HL. International Union against Tuberculosis and Lung Disease, IUATLD. @ in Interventions for tuberculosis control and elimination. Paris: International Union Against Tuberculosis and Lung Disease Paris; (2002).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

