Cost-effectiveness analysis of cadonilimab plus chemotherapy as a first-line treatment option in HER-2-negative advanced gastric cancer
- PMID: 40761936
- PMCID: PMC12318942
- DOI: 10.3389/fpubh.2025.1644176
Cost-effectiveness analysis of cadonilimab plus chemotherapy as a first-line treatment option in HER-2-negative advanced gastric cancer
Abstract
Objective: This study aims to evaluate the cost-effectiveness of using cadonilimab plus chemotherapy compared to chemotherapy in HER-2-negative advanced gastric cancer from the perspective of the Chinese healthcare system.
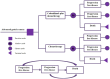
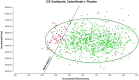
Methods: A cost-effectiveness analysis was conducted utilizing a partitioned survival model to simulate the expected costs and outcomes associated with the treatment of patients with cadonilimab in combination with chemotherapy versus chemotherapy over a 10 years lifetime horizon. Cost data were sourced from published literature and national databases. Data on treatment efficacy, adverse events, and transition probabilities were derived from the phase 3 COMPASSION-15 trial. The WTP threshold in this study was established at 40,343.68 USD per QALY. Sensitivity analyses were performed to evaluate the robustness of the results and assess the impact of variations in key parameters on the cost-effectiveness outcomes.
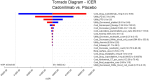
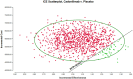
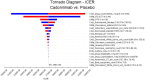
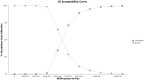
Results: The base case analysis revealed that in all population of randomized patients, treatment with cadonilimab resulted in an incremental gain of 1.08 QALYs compared to chemotherapy, at an incremental cost of 58,862.61 USD. The ICER for this cohort was calculated to be 54,502.42 USD per QALY. In the subgroup of patients with a PD-L1 CPS ≥ 5, patients treated with cadonilimab experienced a greater increase in 1.33 QALYs compared to chemotherapy, at an incremental cost of 35,661.87 USD. The ICER for this subgroup was 26,813.44 USD per QALY. Sensitivity analyses conducted demonstrated the robustness of the results to variations in model inputs and assumptions. Moreover, the probabilistic sensitivity analysis indicated that cadonilimab in combination with chemotherapy had a 4.70 and 93.90% probabilities of being considered cost-effective at a WTP threshold of 40,343.68 USD per QALY for the all randomized patient group and the subgroup of patients with a PD-L1 CPS ≥ 5, respectively.
Conclusion: The addition of cadonilimab to standard chemotherapy for first line treatment of HER-2-negative advanced gastric cancer may not be considered a cost-effective option compared to chemotherapy alone. However, for the subgroup of patients with PD-L1CPS ≥ 5, the ICER was 26,813.44 USD per QALY, indicating that this treatment approach could potentially be deemed cost-effective in China.
Keywords: HER-2-negative; advanced gastric cancer; cadonilimab; chemotherapy; cost-effectiveness analysis.
Copyright © 2025 Zhang, Yang and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- GBD 2017 Stomach Cancer Collaborators . The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. (2020) 5:42–54. doi: 10.1016/S2468-1253(19)30328-0, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

