Mechanisms of T-cell metabolic reprogramming in the microenvironment of acute myeloid leukemia and its therapeutic potential (Review)
- PMID: 40762019
- PMCID: PMC12319392
- DOI: 10.3892/ol.2025.15201
Mechanisms of T-cell metabolic reprogramming in the microenvironment of acute myeloid leukemia and its therapeutic potential (Review)
Abstract
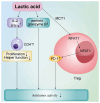
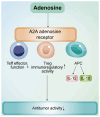
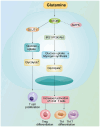
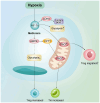
Acute myeloid leukemia (AML) is an aggressive hematological malignancy that is often resistant to conventional therapies. The present narrative review discusses on the role of T cell metabolic reprogramming in the AML tumor microenvironment (TME), which markedly impacts the effectiveness of immunotherapy. The TME of AML, influenced by factors such as high lactic acid (LA) levels, hypoxia and nutrient competition, hampers T cell functions such as glycolysis, lipid metabolism and amino acid metabolism, leading to impaired T cell proliferation and antitumor response. Metabolic waste products, including LA and adenosine, further contribute to the immunosuppressive environment. T cell exhaustion, induced by nutrient deprivation and metabolic dysregulation, serves a key role in the failure of immune responses. Moreover, strategies to modulate T cell metabolism, such as targeting glycolysis and fatty acid oxidation, show promise in enhancing immunotherapy outcomes. The current review also highlights emerging technologies, such as single-cell metabolomics and CRISPR screening, which are critical for identifying metabolic targets and advancing personalized therapies. Despite challenges in translating these findings to clinical settings, understanding T cell metabolism in the AML TME offers new therapeutic avenues for improving patient outcomes.
Keywords: T cells; immunization; tumor immunology.
Copyright: © 2025 Luo et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
