Ponatinib, But Not the New Abl-Kinase Inhibitor Asciminib, Activates Platelets, Leukocytes, and Endothelial Cell TNF Signaling to Induce Atherosclerotic Plaque Inflammation, Myocardial Infarction, and Stroke
- PMID: 40762051
- PMCID: PMC12333468
- DOI: 10.1161/CIRCULATIONAHA.125.073610
Ponatinib, But Not the New Abl-Kinase Inhibitor Asciminib, Activates Platelets, Leukocytes, and Endothelial Cell TNF Signaling to Induce Atherosclerotic Plaque Inflammation, Myocardial Infarction, and Stroke
Abstract
Background: Imatinib, the first Abl-tyrosine kinase inhibitor (TKI), improved leukemia outcomes without cardiovascular side effects. Newer agents, including ponatinib, addressed imatinib resistance, improving cancer remission, but substantially increased arterial thrombotic events, including myocardial infarction (MI) and stroke. The mechanism behind ponatinib-induced thrombosis and the cardiovascular effect of asciminib, a newly approved Abl-TKI, remain unknown.
Methods: The effect of clinically relevant plasma concentrations of imatinib, ponatinib, and asciminib were compared with vehicle in vivo using SR-BI-mut/LDLR-knockout (KO) mice to assess spontaneous MI and stroke risk. The mechanism was interrogated in C57BL/6J mice, assessing leukocyte trafficking and thromboinflammation by intravital microscopy and flow cytometry, respectively, and in ApoE-KO mice, assessing plaque phenotype by flow cytometry and histology. In vitro effects on human umbilical vein endothelial cells (ECs) and human coronary artery ECs were determined by flow cytometry, PCR, and immunoblotting. The role of TNF (tumor necrosis factor) signaling was evaluated by pharmacological inhibition and small interfering RNA knockdown.
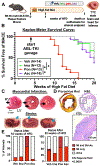
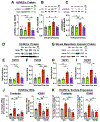
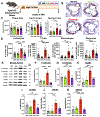
Results: In SR-BI-mut/LDLR-KO mice, ponatinib significantly accelerated death from MI and stroke compared with vehicle, imatinib, and asciminib. In human ECs, only ponatinib increased expression of TNF receptors (TNFRs) and adhesion molecules (P-selectin, ICAM1 [intercellular adhesion molecule 1], and VCAM1 [vascular cell adhesion molecule 1]). Ponatinib rapidly induced TNFR2 membrane trafficking and TNF signaling in human umbilical vein ECs. TNFR inhibition or TNFR2 knockdown prevented ponatinib induction of EC adhesion molecules. In vivo, ponatinib increased mesenteric vessel adhesion molecules, leukocyte rolling and adhesion to vessels, leukocyte and platelet activation, and platelet-leukocyte aggregates. In ApoE-KO mice, ponatinib increased plaque necrotic core and inflammation, consistent with a rupture-prone phenotype. Asciminib-treated mice developed none of these in vitro or in vivo toxicities. In C57BL/6J mice, TNFR inhibition blocked ponatinib-induced mesenteric adhesion molecule expression and leukocyte trafficking, but not platelet-leukocyte aggregation. TNFR blockade prevented ponatinib-induced plaque inflammation in ApoE-KO mice and MI and stroke in SR-BI-mut/LDLR-KO mice.
Conclusions: Ponatinib, a potent anticancer therapy, activates ECs, platelets, and leukocytes, driving plaque inflammation and death from MI and stroke in mice, mirroring clinical cardiotoxicities in patients with cancer. Asciminib did not induce these effects, suggesting it might be a safer option for imatinib-resistant patients with cancer. Inhibition of TNFR-mediated endothelial activation is sufficient to prevent ponatinib-induced major adverse cardiovascular events.
Keywords: asciminib hydrochloride; atherosclerosis; cardio-oncology; endothelial cells; imatinib mesylate; inflammation; ponatinib hydrochloride.
Conflict of interest statement
The authors report no relevant conflicts of interest. Dr Chen is a founder of and owns shares in Satellite Biosciences, a company that is developing cell-based therapies; and Ropirio Therapeutics, a company that is developing pharmaceuticals. Dr Jaffe is a consultant for Boehringer Ingelheim in an unrelated area.
Figures





References
-
- Ryan TD, Bates JE, Kinahan KE, Leger KJ, Mulrooney DA, Narayan HK, Ness K, Okwuosa TM, Rainusso NC, Steinberger J, et al. Cardiovascular Toxicity in Patients Treated for Childhood Cancer: A Scientific Statement From the American Heart Association. Circulation. 2025. doi: 10.1161/CIR.0000000000001308 - DOI - PMC - PubMed
-
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MWN, Silver RT, Goldman JM, Stone RM, et al. Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. New England Journal of Medicine. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

