Development of Enhanced Separation Techniques for Oligonucleotides Utilizing Mixed-Mode Chromatography and 2D-LC/UV/MS Analysis
- PMID: 40762085
- PMCID: PMC12322800
- DOI: 10.1002/jssc.70238
Development of Enhanced Separation Techniques for Oligonucleotides Utilizing Mixed-Mode Chromatography and 2D-LC/UV/MS Analysis
Abstract
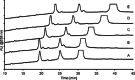
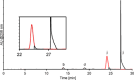
Oligonucleotides are traditionally analyzed using ion-pair reversed-phase liquid chromatography. In this project, we explored an alternative approach utilizing a reversed phase-weak anion exchange mixed-mode column, which combines separation based on both charge and hydrophobicity. Our findings indicate that the mixed-mode column provided stronger retention and superior separation compared to the C18 column, eliminating the need for ion-pairing reagents. Additionally, selectivity can be adjusted using gradient pH and buffer concentration as well as acetonitrile content. However, the use of a phosphate buffer was necessary to ensure adequate retention and separation, despite its incompatibility with MS detection. To address this issue, a 2DLC-UV-qTOF setup with multiple loops was established, where the second-dimension LC, connected to the MS detector, required only an MS-compatible mobile phase. Using a HILIC-type LUNA Omega Sugar column in the second-dimension LC, the MS intensity of the heart-cut from the first dimension was increased 100-fold, allowing for the characterization of the main peak.
Keywords: 2D liquid chromatography; mass spectrometry; mixed‐mode chromatography; oligonucleotide; reversed‐phase/weak anion‐exchange.
© 2025 The Author(s). Journal of Separation Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

