Selective Deletion of NBCe1 in Reactive Astrocytes Attenuates Ischemic Stroke Brain Damage
- PMID: 40762246
- PMCID: PMC12541896
- DOI: 10.1002/glia.70075
Selective Deletion of NBCe1 in Reactive Astrocytes Attenuates Ischemic Stroke Brain Damage
Abstract
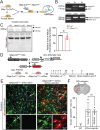
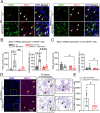
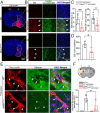
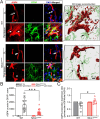
The electrogenic sodium bicarbonate transporter 1 (NBCe1/Slc4a4), predominantly expressed in astrocytes, is important for brain pH regulation and homeostasis. Increased NBCe1 expression in reactive astrocytes has been associated with neuronal degeneration in ischemic stroke. However, the effects of astrocytic NBCe1 inhibition in stroke remain contradictory, and the underlying mechanisms are unclear. Here, we show that wild-type (WT) mice exhibited elevated NBCe1 expression in the peri-lesional regions at 3 days post-stroke. Astrocytic Nbce1 gene deletion in inducible Gfap-Cre ERT2+/-; Nbce1 f/f mice (Nbce1 iΔAstro) resulted in a significant reduction in NBCe1 mRNA and protein expression in astrocytes. Compared to WT stroke mice, Nbce1 iΔAstro mice displayed reduced infarct volume, decreased brain swelling, improved cerebral blood flow, and accelerated neurological function recovery in the 1-5-day acute post-stroke period. Moreover, Nbce1 iΔAstro stroke mice exhibited decreased blood-brain barrier (BBB) permeability, accompanied by preserved perivascular AQP4 polarization, upregulation of Kir4.1 protein expression, and reduced astrocyte domain volume. Importantly, Nbce1 iΔAstro stroke brains revealed an anti-inflammatory cytokine profiling signature, marked by increased TIMP-1 expression. Together, our findings suggest that astrocytic upregulation of pH regulatory protein NBCe1 after stroke contributes to increased BBB permeability, reactive astrogliosis, inflammation, and perivascular AQP4 dysregulation. Targeting astrocytic NBCe1 may represent a promising new therapeutic strategy to mitigate astroglial dysfunction in the post-stroke brain.
Keywords: AQP4; NBCe1; astrocytic end‐feet; brain pH homeostasis; ischemic stroke.
© 2025 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

