The Efficacy of Rosehip Oil Emulsion as a Pro-Healing Herbal Medicine for the Treatment of Induced Mucosal Ulcer (Cell Culture and Experimental Study)
- PMID: 40762679
- PMCID: PMC12327070
- DOI: 10.3290/j.ohpd.c_2158
The Efficacy of Rosehip Oil Emulsion as a Pro-Healing Herbal Medicine for the Treatment of Induced Mucosal Ulcer (Cell Culture and Experimental Study)
Abstract
Purpose: This study aimed to determine the impact of rose hip oil emulsion (ROE) on the healing of oral ulcers. The study first utilised the MTT kit to examine the effect of 20 mg/mL ROE on human gingival fibroblast (HGF) proliferation at the cellular level and its effect in treating oral mucosal ulcers at the experimental level.
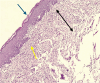
Materials and methods: Sixty-six adult male rats with a chemically induced ulcer in the buccal mucosa. The animals were distributed randomly into two groups: a control group that had not received any treatment and a test group that was treated with topical ROE 3 times per day for 10 days. The samples were obtained on day 3, day 7, and day 10, and then the tissue staining was done using hematoxylin and eosin (H&E) and histomorphometric analysis.
Results: The findings of this study demonstrated from cellular investigations that 20 mg/mL ROE can efficiently stimulate HGF proliferation at 24, 48, and 72 h. The animal study results revealed that ROE could substantially boost the healing of the induced ulcer model by lowering the inflammatory cells and extensively promoting collagen formation within the ulcer site on days 3 and 7.
Conclusion: The topical application of 20 mg/mL ROE possesses anti-inflammatory properties, increasing the epithelium thickness and promoting collagen production and remodelling. Therefore, the rosehip oil emulsion can be considered an effective pro-healing agent that accelerates the healing of oral ulcers.
Keywords: anti-inflammatory activity; oral ulcer; rosehip oil; wound healing.
Figures












References
-
- Ala A, Ebrahimi Bakhtavar H, Shams Vahdati S, Rahmani F, Azargoun M, Ebrahimi BH. Effects of silver sulfadiazine and Adibderm® herbal ointments in treatment of patients with second degree burns: a randomized clinical trial. Trauma Mon. 2018;23:e13396.
-
- Belkhelladi M, Bougrine A. Rosehip extract and wound healing: a review. J Cosmet Dermatol. 2024;23:62–67. - PubMed
-
- Chrubasik C, Roufogalis BD, Müller-Ladner U, Chrubasik S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother Res. 2008;22:725–733. - PubMed
-
- Daels-Rakotoarison DA, Gressier B, Trotin F, Brunet C, Luyckx M, Dine T. Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother Res. 2002;16:157–161. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

