Systematic comparison of methods for offline breath sampling
- PMID: 40762732
- PMCID: PMC12402010
- DOI: 10.1007/s00216-025-06025-5
Systematic comparison of methods for offline breath sampling
Abstract
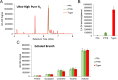
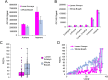
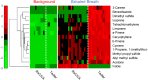
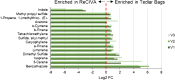
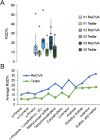
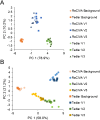
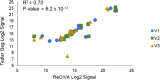
Harnessing the potential of exhaled breath analysis is an emerging frontier in medical diagnostics, given breath is a rich source of volatile organic compound (VOC) biomarkers for different medical conditions. A current downfall in this field, however, is the lack of standardized and widely available methods for offline sampling of exhaled VOCs. Herein, strides are taken toward the standardization of breath sampling in Tedlar bags by exploring several factors that can impact VOC heterogeneity, including tubing material, chemical composition of collection bags, breath fractionation, exhalation volume, and transfer flow rate. After bag-based sampling standardization, performance was benchmarked using two offline breath sampling methods, Tedlar bags and the Respiration Collector for In Vitro Analysis (ReCIVA). Three volunteers from the laboratory with no known respiratory diseases donated ≥ n = 5 samples collected onto adsorption tubes via each method, which were analyzed through thermal desorption (TD) coupled with gas chromatography-mass spectrometry (GC-MS). Data processing revealed a set of 15 highly reliable on-breath VOCs detected across volunteers, and most analytes (except indole) demonstrated higher sensitivity using Tedlar bags. Calculating relative standard deviation (RSD) values showed Tedlar bags were also significantly more reproducible compared to the ReCIVA (p < 0.03). Agreement between the two methods was demonstrated through correlating VOC signals with high statistical significance (R2 = 0.70), indicating both devices are well situated for biomarker discovery applications.
Keywords: Exhaled breath; Gas chromatography-mass spectrometry (GC–MS); Method standardization; Thermal desorption; Volatile organic compounds (VOCs).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Source of biological materials and ethics approval: Biological samples (exhaled breath) were collected from three laboratory volunteers in this study. All the laboratory volunteers consented, and all study procedures abided by the Indiana University Institutional Review Board protocol (IRB # 15542). Competing interests: Mangilal Agarwal has an ongoing collaboration with NANOZ and Scosche Industries to commercialize metal oxide sensors to detect VOC biomarkers, some of which are presented in this work. All other authors report no relevant conflicts of interest.
Figures



References
-
- Boots AW, van Berkel JJ, Dallinga JW, Smolinska A, Wouters EF, van Schooten FJ. The versatile use of exhaled volatile organic compounds in human health and disease. J Breath Res. 2012;6(2):027108. - PubMed
-
- Haworth JJ, Pitcher CK, Ferrandino G, Hobson AR, Pappan KL, Lawson JLD. Breathing new life into clinical testing and diagnostics: perspectives on volatile biomarkers from breath. Crit Rev Clin Lab Sci. 2022;59(5):353–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

