IFNγ in human sepsis: a scoping review
- PMID: 40762872
- PMCID: PMC12325153
- DOI: 10.1186/s13613-025-01534-z
IFNγ in human sepsis: a scoping review
Abstract
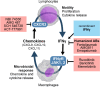
Background: The cytokine IFNγ is released primarily by lymphocytes to initiate and orchestrate immune responses in a broad range of target cells. Whereas immune cells release inflammatory mediators and initiate antimicrobial responses when stimulated by IFNγ, parenchymal cells frequently display increased immunogenicity and incidental cell death. In addition to these well-characterized effects of IFNγ, recent studies disclose a key role of the cytokine in sepsis and organ dysfunction. MAIN: This review summarizes current knowledge on the IFNγ response to infection and attempts to relate the IFNγ response to endophenotypes of sepsis in the human host. Both, excessive pro-inflammatory responses with high IFNγ and downstream mediators, such as chemokines (CXCL9), as well as immunosuppression with low IFNγ levels are associated with unfavorable outcomes in sepsis. Pilot studies suggested beneficial effects of recombinant IFNγ in counteracting immunosuppression associated with low IFNγ levels. On the other hand, IFNγ may induce macrophages to release chemokines CXCL9, 10, and 11 to attract B and T lymphocytes to the sites of infection. Downstream induction of CXCL9 (but not of CXCL10 and 11) occurring in a subset of patients with high IFNγ levels has been shown to correlate with the hyper-inflammatory phenotype of sepsis. Both, high- and low-expressing IFNγ phenotypes of sepsis, might be related to nucleotide polymorphisms of the human IFNγ gene.
Conclusion: Association of IFNγ activity states with sepsis outcome renders this key regulatory protein of immunity a top candidate for theranostic interventions in a "personalized medicine approach" to infection and sepsis, especially when combined with additional biomarkers, such as CXCL9, reflecting or even mediating maladaptive downstream actions.
Keywords: Hyperinflammation; IFNγ; Immunostimulatory therapy; Immunosuppression; Immunosuppressive therapy; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and materials: Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–22. - PubMed
-
- Roquilly A, McWilliam HEG, Jacqueline C, Tian Z, Cinotti R, Rimbert M, Wakim L, Caminschi I, Lahoud MH, Belz GT, Kallies A, Mintern JD, Asehnoune K, Villadangos JA. Local modulation of antigen-presenting cell development after resolution of pneumonia induces long-term susceptibility to secondary infections. Immunity. 2017;47(135–147):e135. - PubMed
-
- Roquilly A, David G, Cinotti R, Vourc’h M, Morin H, Rozec B, Retiere C, Asehnoune K. Role of IL-12 in overcoming the low responsiveness of NK cells to missing self after traumatic brain injury. Clin Immunol. 2017;177:87–94. - PubMed
-
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

