Aging Adipose-Derived Mesenchymal Stem Cells, Cultured on a Native Young Extracellular Matrix, Are Protected From Senescence and Apoptosis Along With Increased Expression of HLA-DR and CD74 Associated With PI3K Signaling
- PMID: 40763035
- PMCID: PMC12419859
- DOI: 10.1111/acel.70165
Aging Adipose-Derived Mesenchymal Stem Cells, Cultured on a Native Young Extracellular Matrix, Are Protected From Senescence and Apoptosis Along With Increased Expression of HLA-DR and CD74 Associated With PI3K Signaling
Abstract
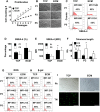
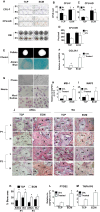
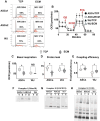
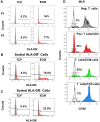
Older adults are the primary population for cell-based therapies for age-related diseases, but the efficacy of administering autologous mesenchymal stem cells (MSCs) is impaired due to biological aging. In the present study, we cultured aging adipose (AD)-derived MSCs from > 65-year-old donors on extracellular matrix (ECM) synthesized by human amniotic fluid-derived pluripotent stem cells (ECM Plus) versus tissue culture plastic (TCP) and hypothesized that ECM Plus provided an ideal "young" microenvironment for reactivating and preserving early-stage progenitor cells within aging AD-MSCs. To test our hypothesis, we serially sub-cultured aging AD-MSCs on ECM Plus or TCP and characterized the cells both phenotypically and functionally, and then analyzed the cells at the single-cell transcriptomic level for the mechanisms that control cell fate. The results showed that the maintenance of aging AD-MSCs on ECM Plus significantly restored their quantity and quality. The mechanisms responsible for these effects were associated with a remarkable up-regulation of intracellular CD74 when cells were maintained on ECM Plus compared to TCP, which triggered activation of the phosphoinositide-3-kinase (PI3K) pathway as a key modulator of cell survival (anti-apoptosis) and suppression of cellular senescence. Moreover, AD-MSCs maintained on ECM Plus increased their expression of HLA-DR and stimulated T cell activity. These findings challenge the "immune privilege" of allogeneic MSCs as a universal source for MSC-based therapies. The present study leads to a new paradigm for treating age-related diseases: serial administration of rejuvenated autologous MSCs, which may not only replace aged MSCs but also gradually reverse the aged microenvironment.
Keywords: CD74; MHC class II; aged MSCs; autologous stem cell therapies; immunogenicity; young microenvironment.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Chen is a Board member and shareholder in StemBioSys Inc. (San Antonio, TX). All other authors have no financial or competing interests to declare.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

