RSV vaccination uptake among adults aged 60 years and older in the United States during the 2023-2025 vaccination seasons
- PMID: 40763207
- PMCID: PMC12326572
- DOI: 10.1080/21645515.2025.2535755
RSV vaccination uptake among adults aged 60 years and older in the United States during the 2023-2025 vaccination seasons
Abstract
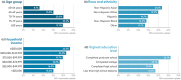
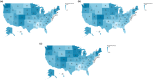
Older adults and adults with certain chronic conditions are at increased risk for severe respiratory syncytial virus (RSV) disease. In 2023, RSV vaccines first became available in the United States (US) for adults aged ≥60 years. This retrospective database analysis evaluated RSV vaccination uptake from August 2023-February 2025 using IQVIA's open-source pharmacy (LRx) and medical (Dx) claims data. The study included US adults aged ≥60 years with ≥1 claim in 2023. For those without RSV vaccination in 2023, ≥1 claim was also required between January 2024-February 2025. Uptake was assessed as the number and percentage of eligible adults who received an RSV vaccine during the study period. Multivariable logistic regression modeling explored factors associated with RSV vaccination. Nearly 12.8 million adults aged ≥60 years (16.4%) received RSV vaccination between August 2023-February 2025. Uptake generally increased with age and was higher among those with ≥1 potential risk factor for severe RSV disease. Disparities in uptake were observed by race, ethnicity, and other social determinants of health. In multivariable analyses, odds of RSV vaccination were nearly 24 times higher for those who received ≥1 non-RSV vaccine from August 2023-February 2025 versus those who had not. Despite the increased risk of severe RSV disease among older adults and those with certain risk factors, relatively limited RSV vaccination uptake was observed during the 2023-2025 seasons, with disparities observed. Additional efforts are needed to support RSV prevention among those at highest risk and to ensure equitable access to vaccination.
Keywords: RSVpreF vaccine; United States; Vaccination uptake; adjuvanted RSVPreF3 vaccine; coadministration; disparities; older adults; respiratory syncytial virus.
Plain language summary
What is the context?Respiratory syncytial virus (RSV) can result in severe illness in adults aged 60 years and older, as well as adults with certain health conditions.RSV vaccines for use in adults aged 60 years and older became available in the United States (US) in 2023.What is new?During the first two seasons of vaccine availability, only 16.4% of US adults aged 60 years and older received an RSV vaccination (August 2023–February 2025).The percentage of older adults receiving RSV vaccination was relatively low, even among adults with health conditions that place them at increased risk of severe RSV illness.Differences in uptake were found by race, ethnicity, and other patient characteristics.What is the impact?Additional efforts are needed to ensure that adults aged 60 years and older are able to receive RSV vaccination.
Conflict of interest statement
EL and DS are employees of and hold financial equities in GSK. CM, MY, and CC are employees of IQVIA, which received funding from GSK for the conduct of this study. CM is a Pfizer stockholder. The authors declare no other financial and non-financial relationships and activities.
Figures



References
-
- CDC . RSV in older adults; 2025. [accessed 2025 Jun 17]. https://www.cdc.gov/rsv/older-adults/index.html.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
