Tumor-infiltrating nociceptor neurons promote immunosuppression
- PMID: 40763210
- PMCID: PMC12428838
- DOI: 10.1126/scisignal.ads7889
Tumor-infiltrating nociceptor neurons promote immunosuppression
Abstract
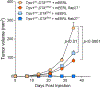
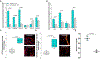
Small extracellular vesicles (sEVs) released from tumors recruit nociceptor neurons to the tumor bed. Here, we found that ablating these neurons in mouse models of head and neck carcinoma and melanoma reduced the infiltration of myeloid-derived suppressor cells (MDSCs). Moreover, sEV-deficient tumors failed to develop in mice lacking nociceptor neurons. We investigated the interplay between tumor-infiltrating nociceptors and immune cells in head and neck squamous cell carcinoma (HNSCC) and melanoma. Upon exposure to cancer-derived sEVs, mouse dorsal root ganglion (DRG) neurons secreted increased amounts of substance P, IL-6, and injury-associated neuronal markers. Patient-derived sEVs sensitized DRG neurons to capsaicin, implying enhanced nociceptor responsiveness. Furthermore, nociceptors cultured with sEVs induced an immunosuppressed state in CD8+ T cells. Incubation with conditioned medium from cocultures of neurons and cancer cells resulted in increased expression of markers of MDSCs and suppressive function in primary bone marrow cells, and the combination of neuron-conditioned medium and cancer sEVs promoted checkpoint receptor expression on T cells. Together, these findings reveal that nociceptor neurons facilitate CD8+ T cell exhaustion and bolster MDSC infiltration into HNSCC and melanoma. Consequently, targeting nociceptors may provide a strategy to disrupt detrimental neuroimmune cross-talk in cancer and potentiate antitumor immunity.
Conflict of interest statement
Figures

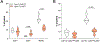

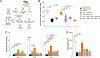


Update of
-
TUMOR-INFILTRATING NOCICEPTOR NEURONS PROMOTE IMMUNOSUPPRESSION.bioRxiv [Preprint]. 2024 Aug 26:2024.08.23.609450. doi: 10.1101/2024.08.23.609450. bioRxiv. 2024. Update in: Sci Signal. 2025 Aug 5;18(898):eads7889. doi: 10.1126/scisignal.ads7889. PMID: 39253487 Free PMC article. Updated. Preprint.
References
-
- Cillo AR, Kurten CHL, Tabib T, Qi Z, Onkar S, Wang T, Liu A, Duvvuri U, Kim S, Soose RJ, Oesterreich S, Chen W, Lafyatis R, Bruno TC, Ferris RL, Vignali DAA. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity. 2020;52(1):183–99 e9. Epub 20200107. doi: 10.1016/j.immuni.2019.11.014. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

