Double trouble: cytosolic and nuclear IKKα in cancer
- PMID: 40763800
- PMCID: PMC12324875
- DOI: 10.1098/rsob.240375
Double trouble: cytosolic and nuclear IKKα in cancer
Abstract
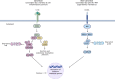
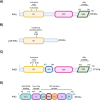
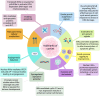
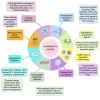
IκB kinase alpha (IKKα) is a serine/threonine kinase originally known for its role in nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling, which integrates inflammatory processes and cancer. IKKα can function within the IKK complex in canonical NF-κB signalling, alongside its homologous family member, IKKβ, and regulatory subunit IKKγ (NEMO). However, a key role for IKKα is its ability to promote non-canonical NF-κB signalling. Additionally, the dynamic ability of IKKα to shuttle between the cytosol and nucleus, mediates NF-κB-independent effects which further its role in inflammation and tumour progression. More recently, an endosomal-generated, nuclear-active IKKα isoform, p45-IKKα has been discovered and implicated in cancer and chemoresistance. This review focuses on current knowledge of the complex and intricate roles of nuclear and cytosolic IKKα in promoting tumour progression. By highlighting the molecular roles of IKKα in several cancer subtypes, and its integral roles in many of the hallmarks of cancer throughout this review, we highlight the therapeutic potential of IKKα as a future anti-cancer drug target.
Keywords: IKKα; NF-κB; cancer; cytosolic; nuclear.
Conflict of interest statement
We declare we have no competing interests.
Figures
Similar articles
-
Regulatory subunit NEMO promotes polyubiquitin-dependent induction of NF-κB through a targetable second interaction with upstream activator IKK2.J Biol Chem. 2022 May;298(5):101864. doi: 10.1016/j.jbc.2022.101864. Epub 2022 Mar 24. J Biol Chem. 2022. PMID: 35339487 Free PMC article.
-
A 36-base hairpin within lncRNA DRAIC, which is modulated by alternative splicing, interacts with the IKKα coiled-coil domain and inhibits NF-κB and tumor cell phenotypes.J Biol Chem. 2025 Jun;301(6):110172. doi: 10.1016/j.jbc.2025.110172. Epub 2025 May 2. J Biol Chem. 2025. PMID: 40320073 Free PMC article.
-
IL-1β stimulates a novel, IKKα -dependent, NIK -independent activation of non-canonical NFκB signalling.Cell Signal. 2023 Jul;107:110684. doi: 10.1016/j.cellsig.2023.110684. Epub 2023 Apr 18. Cell Signal. 2023. PMID: 37080443
-
Decoding NF-κB: nucleocytoplasmic shuttling dynamics, synthetic modulation and post-therapeutic behavior in cancer.Mol Biol Rep. 2025 Aug 7;52(1):804. doi: 10.1007/s11033-025-10917-1. Mol Biol Rep. 2025. PMID: 40775128 Review.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
References
-
- Hanahan D. 2022. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46. ( 10.1158/2159-8290.cd-21-1059) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

