Nationwide survey of coeliac disease serology testing in the UK
- PMID: 40764046
- PMCID: PMC12336569
- DOI: 10.1136/bmjgast-2025-001900
Nationwide survey of coeliac disease serology testing in the UK
Abstract
Objective: Recent evidence supports diagnosing coeliac disease without biopsy in patients with significantly elevated tissue transglutaminase (IgA-tTG) antibodies. However, the implementation of this no-biopsy approach relies on accurate and consistent serological testing across laboratories. In this nationwide survey, we aimed to evaluate the availability and variability of coeliac disease testing across the UK.
Methods: We conducted a cross-sectional telephone survey of biomedical scientists and laboratory managers from National Health Service trusts and health boards across England, Wales, Scotland, and Northern Ireland. Data collected included assay types, reporting methods, upper limit of normal (ULN) thresholds, turnaround times, total IgA testing, and anti-endomysial antibodies (EMAs) availability.
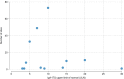
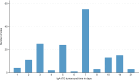
Results: A total of 356 sites were approached, with a 96% response rate (n=342). Of responding sites, 177 performed coeliac serology tests in-house, while 165 transferred samples externally. Among sites performing tests, 12 different IgA-tTG assays were identified, with considerable variability in ULN thresholds ranging from 3 to 30 IU/mL, even within laboratories using the same assays. The median turnaround time for IgA-tTG results was 7 days (range 1-21 days). Only 43% of laboratories routinely measured total IgA when IgA-tTG was requested. EMA testing was available in 83% of laboratories.
Conclusion: Significant variability exists in coeliac serology testing across UK laboratories which poses a challenge for the implementation of the no-biopsy approach in clinical practice. Efforts to standardise serological testing are urgently needed. Until such standardisation is achieved, local assay validation remains critical.
Keywords: CELIAC DISEASE; SMALL BOWEL; SMALL BOWEL DISEASE.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MGS and HAP received speaker fees from Thermo Fisher. The other authors declare no competing interests.
Figures


References
-
- Conrad N, Misra S, Verbakel JY, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401:1878–90. doi: 10.1016/S0140-6736(23)00457-9. - DOI - PubMed
-
- Schiepatti A, Maimaris S, Raju SA, et al. Persistent villous atrophy predicts development of complications and mortality in adult patients with coeliac disease: a multicentre longitudinal cohort study and development of a score to identify high-risk patients. Gut. 2023;72:2095–102. doi: 10.1136/gutjnl-2023-329751. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
