Functional avidity of anti-B7H3 CAR-T constructs predicts antigen density thresholds for triggering effector function
- PMID: 40764385
- PMCID: PMC12325614
- DOI: 10.1038/s41467-025-61427-4
Functional avidity of anti-B7H3 CAR-T constructs predicts antigen density thresholds for triggering effector function
Abstract
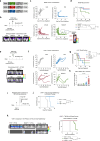
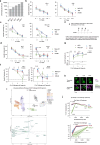
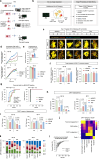
Chimeric Antigen receptor T cell (CAR-T) treatments for solid cancers have been compromised by limited expansion and survival in the tumor microenvironment following interaction with antigen-expressing target cells. Using B7H3 as a model antigen with broad clinical applicability, we evaluate the relationship between the antibody/antigen affinity of three clinical candidate binders and the three following characteristics: cellular avidity, duration of sustained cytotoxicity in tumoroid re-stimulation assays, and in vivo anti-tumoral responses. Next, BEHAV3D video microscopy is used to assess CAR-T cell interaction with tumor cells at single cell resolution. These data are consistent with a threshold avidity of CAR-T / tumor cell interaction and target cell B7H3 expression level, where enhanced functionality is characterized by longer cumulative CD8+ CAR-T / tumor target interaction times, CAR-T cell expansion and sustained tumor control. Lower checkpoint receptor expression does not correlate with enhanced anti-tumor function. These results provide further insights into design of anti-B7H3 CAR-T cells for antigen-dim cell targeting, and avoidance of antigen-dim tumor relapse.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.Ba, J.A., K.C., K.B., and M.Bu hold patents in CAR-T technology development, including a pending patent for the TE9 anti-B7H3 binder. J.A. holds founder shares in Autolus. R.N., R.R., and D.S. were employed by Lumicks at the time of performing the experiments. The remaining authors declare no competing interests.
Figures




References
-
- Albelda, S. M. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat. Rev. Clin. Oncol.21, 47–66 (2024). - PubMed
-
- Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov.8, 1219–1226 (2018). - PubMed
-
- Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature614, 635–648 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

