Convergent activation of the integrated stress response and ER-mitochondria uncoupling in VAPB-associated ALS
- PMID: 40764463
- PMCID: PMC12423299
- DOI: 10.1038/s44321-025-00279-3
Convergent activation of the integrated stress response and ER-mitochondria uncoupling in VAPB-associated ALS
Abstract
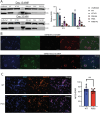
Vesicle-associated membrane protein-associated protein-B (VAPB) is an endoplasmic reticulum (ER) membrane-bound protein. The P56S mutation in VAPB causes a dominant, familial form of amyotrophic lateral sclerosis (ALS). However, the mechanism by which this mutation leads to motor neuron (MN) degeneration remains unclear. Utilizing inducible pluripotent stem cell (iPSC)-derived MNs expressing either wild-type (WT) or P56S VAPB, we demonstrate that the mutant protein reduces neuronal firing and disrupts ER-mitochondria-associated membranes (ER MAMs), with a time-dependent decline in mitochondrial membrane potential (MMP), hallmarks of MN pathology. These findings were validated in patient-derived iPSC-MNs. Additionally, VAPB P56S MNs show increased susceptibility to ER stress, elevated expression of the Integrated Stress Response (ISR) regulator ATF4 under stress, and reduced global protein synthesis. Notably, pharmacological ISR inhibition using ISRIB rescued ALS-associated phenotypes in both VAPB P56S and patient-derived iPSC-MNs. We present the first evidence that the VAPB P56S mutation activates ISR signaling via mitochondrial dysfunction in human MNs. These findings support ISR modulation as a strategy for ALS intervention and highlight the need for patient stratification in clinical trials.
Keywords: ALS (Amyotrophic Lateral Sclerosis); ER-MAM (Endoplasmic Reticulum Mitochondria Associated Membrane); ISR (Integrated Stress Response); Neurodegeneration; VAPB ((Vesicle Associated Membrane Protein Associated Protein B).
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. HCM and ARM serve on the Scientific Advisory Board of Axion BioSystems, whose technology was used in this study. The authors declare no competing interests.
Figures














References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

