Matrix-M adjuvant triggers inflammasome activation and enables antigen cross-presentation through induction of lysosomal membrane permeabilization
- PMID: 40764493
- PMCID: PMC12325594
- DOI: 10.1038/s41541-025-01243-5
Matrix-M adjuvant triggers inflammasome activation and enables antigen cross-presentation through induction of lysosomal membrane permeabilization
Abstract
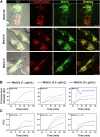
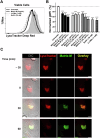
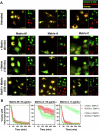
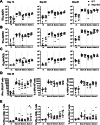
Matrix-M® adjuvant, containing saponins, delivers a potent adjuvant effect and good safety profile. Given that Matrix-M is composed of Matrix-A and Matrix-C particles, comprising different saponin fractions, understanding their distinct roles can provide deeper insight into the mechanism of action of Matrix-M and guide future applications. Here, we demonstrate that the antigen and Matrix-M, Matrix-A, or Matrix-C colocalize in lysosomes following uptake by bone marrow-derived dendritic cells. Matrix-M, Matrix-A, and Matrix-C induce lysosomal membrane permeabilization (LMP), but Matrix-C shows the highest LMP potential. LMP is required for interleukin (IL)-1β and IL-18 secretion in vitro. In vivo, a robust adjuvant effect of Matrix-M, Matrix-A, and Matrix-C is observed, both in the presence and absence of the NLRP3 inflammasome. LMP induced by Matrix-M, as well as Matrix-A and Matrix-C, also enables antigen cross-presentation. Thus, Matrix-induced LMP explains the capability of Matrix-M-adjuvanted protein vaccines to induce CD8+ T-cell responses.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: B.Z., B.C., E.S., P.Ö., E.A., I.L.O., L.L., J.A., P.H., J.B., K.L.B., A.E.P., L.S. and C.L.A. are current or former employees of Novavax AB. E.B. and N.H. were contractors to Novavax AB at the time of manuscript preparation. B.Z., B.C., P.Ö., E.A., I.L.O., L.L., J.A., P.H., J.B., K.L.B., A.E.P. and L.S. are shareholders and/or optionees of Novavax, Inc. J.E. and M.E.S. have no competing interests to declare.
Figures



Similar articles
-
T-bet expressing Tr1 cells driven by dietary signals dominate the small intestinal immune landscape.bioRxiv [Preprint]. 2025 Jul 4:2025.06.30.662190. doi: 10.1101/2025.06.30.662190. bioRxiv. 2025. PMID: 40747421 Free PMC article. Preprint.
-
Inflammasome Activation and IL-1β Release Triggered by Nanosecond Pulsed Electric Fields in Murine Innate Immune Cells and Skin.J Immunol. 2024 Jan 15;212(2):335-345. doi: 10.4049/jimmunol.2200881. J Immunol. 2024. PMID: 38047899 Free PMC article.
-
The sesquiterpene lactone estafiatin exerts an anti-inflammatory effect against Mycobacterium abscessus infection by regulating interleukin-1 beta production.Phytomedicine. 2025 Jul 22;146:157080. doi: 10.1016/j.phymed.2025.157080. Online ahead of print. Phytomedicine. 2025. PMID: 40729891
-
Immunomodulatory potential of dietary soybean-derived saponins.J Anim Sci. 2024 Jan 3;102:skae349. doi: 10.1093/jas/skae349. J Anim Sci. 2024. PMID: 39529449 Review.
-
B cell antigens: A key to optimizing CAR-T cell therapy.Int Rev Immunol. 2025 Jun 19:1-28. doi: 10.1080/08830185.2025.2515839. Online ahead of print. Int Rev Immunol. 2025. PMID: 40537997 Review.
References
-
- Datoo, M. S. et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect. Dis.22, 1728–1736 (2022). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

