Demographics and baseline disease characteristics of UK patients within the global aHUS registry
- PMID: 40764536
- PMCID: PMC12323274
- DOI: 10.1186/s12882-025-04321-x
Demographics and baseline disease characteristics of UK patients within the global aHUS registry
Abstract
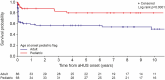
Atypical haemolytic uraemic syndrome (aHUS) is a rare kidney disease characterized by thrombotic microangiopathy. This study presents the first analysis of UK patients enrolled in the Global aHUS Registry, focusing on patient characteristics and disease natural history prior to treatment initiation (n = 172; 74 paediatric, 98 adult). Mean age at first aHUS manifestation was 23.6 years overall (4.9 years for paediatric patients, 37.8 years for adults). Additional thrombotic microangiopathy events occurred in 57.0% of patients between initial clinical suspicion and registry enrolment. Potential precipitating factors were recorded in 14.0% of patients. Of 115 patients at active sites, 90.4% had genetic data recorded, with 73.8% undergoing "complete" genetic testing (results entered for C3, CD46, CFH, CFB and CFI, as a minimum). Of those with genetic data available, 52.9% had an identified pathogenic variant. Gastrointestinal involvement was the most common extra-renal manifestation, presenting in 22.2% of patients. End-stage kidney disease (ESKD) was present in 8.7% at baseline. ESKD-free survival probability at five years was 0.80 for paediatric patients and 0.57 for adults. ESKD-free survival was negatively influenced by CFH, C3, or CFI variants. This study highlights the historically poor prognosis for untreated patients with aHUS. The UK population of the Global aHUS Registry represents a valuable research cohort with comprehensive demographic data and high genetic characterization. These findings underscore the importance of early aHUS identification and intervention to prevent ESKD and improve patient outcomes.
Keywords: Atypical haemolytic-uraemic syndrome (aHUS); Genetics; Prognosis; Registry.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Human ethics and consent to participate: The aHUS Registry is approved through the Research Ethics Committee (REC) North East; Newcastle and North Tyneside 2, chaired by Professor Barry Hirst. The REC approvals are provided to the individual participating sites in the Registry. The Registry is conducted in accordance with International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki. Written informed consent was provided by patients or their parents or guardians, as deemed applicable by institutional review boards or the ethics committee. Consent for publication: Not Applicable. Competing interests: RDG has received several honoraria from Alexion for lectures on aHUS and serving on advisory boards. SDM and AW have no disclosures relating to this manuscript. MSc has received honoraria and a research grant from Alexion. DPG, SG, MSh and NSS have received honoraria from Alexion. CB and TEC are current employees of Alexion, AstraZeneca Rare Disease. IAD, at the time of writing and reviewing, was an employee of AstraZeneca Rare Disease.
Figures

References
-
- Kavanagh D, Richards A, Atkinson J. Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med. 2008;59:293–309. - PubMed
-
- Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Fremeaux-Bacchi V, Kavanagh D, Nester CM, Noris M, Pickering MC, Rodriguez de Cordoba S, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2017;91(3):539–51. - PubMed
-
- Laurence J, Haller H, Mannucci PM, Nangaku M, Praga M, Rodriguez de Cordoba S. Atypical hemolytic uremic syndrome (aHUS): essential aspects of an accurate diagnosis. Clin Adv Hematol Oncol. 2016;14(Suppl 11):2–15. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

