Chorion tissue- and plasma-derived extracellular vesicles exhibit superior anti-inflammatory and chondroprotective effects
- PMID: 40764571
- PMCID: PMC12326873
- DOI: 10.1186/s13287-025-04542-9
Chorion tissue- and plasma-derived extracellular vesicles exhibit superior anti-inflammatory and chondroprotective effects
Abstract
Background: Extracellular vesicles (EVs) are the foundation of modern regenerative medicine using a cell-free approach. While current research mainly explores EVs from biological fluids and cell culture supernatants, tissue-derived EVs hold great promise, but remain largely underexplored. Since healthy placental tissues such as the chorion are widely available after full-term delivery, ethically unobjectionable, and possess exceptional regenerative potential, we sought to compare the biological effects of EVs derived directly from chorion tissue with those from chorion-derived mesenchymal stromal cell EVs and plasma EVs.
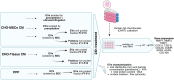
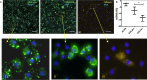
Method: We compared the biological impact of EVs from various sources (chorion tissue CHO-Ti, MSCs from chorion CHO-MSC and platelet-poor plasma PPP) and isolated by various techniques on the gene expression of osteoarthritic chondrocytes. Additionally, we assessed the effect of enriched soluble proteins of CHO-MSC and CHO-Ti secretome vs. their EVs. EVs were characterized by particle number and size (NTA), protein content (BCA assay) and immunophenotype (flow cytometry). Changes in gene expression of chondrocytes were quantified by RT-qPCR.
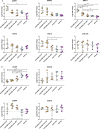
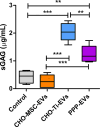
Results: CHO-Ti-EVs and PPP-EVs showed particularly beneficial effect on the inflammatory process, with their biological impact surpassing that of CHO-MSC-EVs. Chondroprotective markers COL2A and ACAN were robustly upregulated by CHO-Ti-EVs and PPP-EVs but showed only modest or variable increases with CHO-MSC-EVs. COMP expression, however, was specifically enhanced by CHO-MSC-derived components. Furthermore, our results also indicate that the therapeutic properties of the CHO-Ti secretome are exclusively linked to EVs. Among CHO-MSC-EVs, purification combined with UC resulted in the highest purity, however EVs purified by SEC presented a more favourable surface marker profile and better biological effects. The observed variability suggests that different EV preparations harbour distinct subpopulations that influence regulatory pathways differently and highlight the importance of EV source and isolation methodology in determining biological activity.
Conclusion: CHO-Ti-EVs showed promising effects on cartilage regeneration and inflammation modulation, suggesting they may represent a viable alternative to plasma- and CHO-MSC-EVs. Moreover, the chorion represents a readily accessible and abundant source of perinatal tissue obtainable non-invasively after full-term delivery, further supporting the translational potential of CHO-Ti-EVs.
Keywords: Cell-free therapy; Chondrocyte; Chorion tissue; Extracellular vesicles; MSC-EVs; Platelet-poor plasma; Tissue-derived EVs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the P. J. Safarik University and L. Pasteur University Hospital in Kosice, Slovakia. Title of the approved projects: ID 2023/EK/06026: Extracellular vesicles from mesenchymal stem cells — a new therapeutic strategy in the treatment of inflammatory joint diseases. Date of approval: 23rd May, 2023. ID 2023/EK/06028: Potential use of circulating extracellular vesicles in the diagnosis of osteoarthritis. Date of approval: 19st June, 2023. Informed consent was obtained from all individual participants included in the study. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, Zhou J, Heng BC, Zou XH, Ouyang H, Liu H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189. 10.1186/s13287-017-0632-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

