Preparation and immunogenicity studies of NvIBDV VP2-ferritin nanoparticles
- PMID: 40764574
- PMCID: PMC12326652
- DOI: 10.1186/s12917-025-04914-6
Preparation and immunogenicity studies of NvIBDV VP2-ferritin nanoparticles
Abstract
Background: Infectious bursal disease (IBD), caused by infectious bursal disease virus (IBDV), is a highly contagious disease that is prevalent worldwide and poses a significant threat to the poultry industry. While commercially available vaccines are used for prevention, IBD outbreaks remain frequent.
Objective: The continuous mutation of virulent strains and their ability to evade traditional vaccine protection complicate IBD control, which necessitates the development of novel vaccines and a deeper understanding of viral mutation mechanisms.
Method: Utilizing the self-assembly capability of ferritin (Fe), the hypervariable region (HVR) protein of a novel variant IBDV (NvIBDV) VP2 was displayed on the ferritin shell, forming regular nanoparticles. The full-length NvIBDV VP2 protein and the NvIBDV VP2-HVR-Fe fusion protein were prokaryotically expressed in E. coli and purified to prepare a VP2 protein vaccine and a VP2-Fe nanoparticle vaccine. An inactivated NvIBDV vaccine served as a control for evaluating immunogenicity and protection.
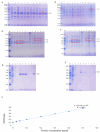
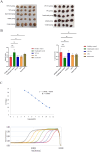
Results: Recombinant prokaryotic expression vectors pET-VP2-Fe (encoding VP2-HVR-Fe) and pET-VP2 (encoding full-length VP2) were successfully constructed. Soluble VP2-Fe and VP2 proteins were expressed and purified. Electron microscopy confirmed the formation of a cage-like nanoparticle structure for VP2-Fe. Immunization of SPF chickens with NvIBDV VP2-Fe nanoparticles induced a robust immune response characterized by high antibody titers and a significantly high protection rate against viral challenge.
Conclusion: The successfully constructed recombinant subunit nanoparticle vaccine, which displays the NvIBDV VP2 HVR on ferritin, effectively increased the antibody titer and provided superior immune protection. This approach offers a feasible strategy for developing novel IBDV subunit vaccines.
Keywords: Ferritin; Infectious bursal disease virus; Nanoparticles; Novel mutant strain; Subunit vaccine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Shanxi Agricultural University, China, and the animal study was reviewed and approved by the Animal Ethical Committee of Shanxi Agricultural University, China. Approval No. SXAU-EAW-2024 C.NR.012026285. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Liao Jiedan C, Yuming Z. Research progress on molecular pathogenesis of infectious bursal disease virus [in chinese]. China Poult. 2018;40(05):1–5.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

