Melatonin and angiogenesis potential in stem cells
- PMID: 40764577
- PMCID: PMC12326606
- DOI: 10.1186/s13287-025-04531-y
Melatonin and angiogenesis potential in stem cells
Abstract
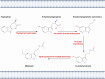
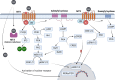
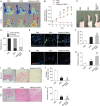
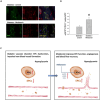
Ischemic diseases, especially coronary artery diseases and myocardial infarction, are the leading cause of human death in the clinical setting. Unfortunately, most of the available clinical interventions can partially restore the function of ischemic myocardium, resulting in the progression of chronic heart failure.The induction of vascular tissue formation, hereafter known as angiogenesis, can provide blood perfusion and prevent the expansion of ischemia-related pathologies. In recent years, the discovery and advent of multiple stem cells into human regenerative medicine have led to the alleviation of certain end-stage pathological conditions via direct differentiation into the mature and functional cells or secretion of various cytokines and angiogenesis factors in a paracrine manner. Melatonin (mel) is a natural molecule with direct and indirect pleiotropic actions on different biological phenomena. This neurohormone is primarily known for its antioxidant, tumoricidal, and anti-inflammatory actions in several pathological conditions. Whether and how mel regulates the angiogenesis behavior of stem cells is currently under debate. Here, we collected and evaluated recent data related to the angiogenic properties of mel on stem cells. Data from the present article may help us in the development of new therapeutic regimes in patients with ischemic conditions.
Keywords: Cell differentiation; Melatonin; Neovascularization; Paracrine communication; Stem cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: No human and/or animal samples were used in the current manuscript. The study was registered as titled “pro-angiogenesis/anti-angiogenesis capacity of melatonin on stem cells under ischemic conditions.” to the research ethics committees of the vice-chancellor in research affairs, tabriz university of medical sciences. Consent for publication: Not applicable. Competing interests: None declared.
Figures


References
-
- Pham VA, et al. Myocardial infarction model induced by isoproterenol in rats and potential cardiovascular protective effect of a nattokinase-containing hard capsule. Phytomedicine Plus. 2023;3(3): 100472.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

