Vertical transmission of chronic wasting disease in free-ranging white-tailed deer populations
- PMID: 40764633
- PMCID: PMC12325800
- DOI: 10.1038/s41598-025-12727-8
Vertical transmission of chronic wasting disease in free-ranging white-tailed deer populations
Abstract
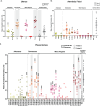
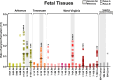
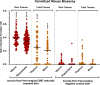
Chronic wasting disease (CWD) is a fatal neurodegenerative disease affecting cervids across North America, Northern Europe, and Asia. Disease transmission among cervids has historically been attributed to direct animal-to-animal contact with 'secreta' (saliva, blood, urine, and feces) containing the infectious agent, and indirect contact with the agent shed to the environment in these bodily components. Mounting evidence provides another mechanism of CWD transmission, that from mother-to-offspring, including during pregnancy (vertical transmission). Here we describe the detection of the infectious CWD agent and prion seeding in fetal and reproductive tissues collected from healthy-appearing free-ranging white-tailed deer (Odocoileus virginianus) from multiple U.S. states by mouse bioassay and in vitro prion amplification assays. This is the first report of the infectious agent in multiple in utero derived fetal and maternal-fetal reproductive tissues, providing evidence that CWD infections are propagated within gestational fetal tissues of white-tailed deer populations. This work confirms previous experimental and field findings in several cervid species supporting vertical transmission as an additional mechanism of CWD transmission and may help to further explain the facile dissemination of this disease among captive and free-ranging cervid populations.
Keywords: Odocoileus virginianus; Cervid; Chronic wasting disease; Prion disease; Vertical transmission; in utero transmission.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Update of
-
Vertical transmission of chronic wasting disease in free-ranging white-tailed deer populations.bioRxiv [Preprint]. 2025 Jan 27:2025.01.24.634834. doi: 10.1101/2025.01.24.634834. bioRxiv. 2025. Update in: Sci Rep. 2025 Aug 5;15(1):28553. doi: 10.1038/s41598-025-12727-8. PMID: 39974995 Free PMC article. Updated. Preprint.
Similar articles
-
Vertical transmission of chronic wasting disease in free-ranging white-tailed deer populations.bioRxiv [Preprint]. 2025 Jan 27:2025.01.24.634834. doi: 10.1101/2025.01.24.634834. bioRxiv. 2025. Update in: Sci Rep. 2025 Aug 5;15(1):28553. doi: 10.1038/s41598-025-12727-8. PMID: 39974995 Free PMC article. Updated. Preprint.
-
Infectious Prions in the Pregnancy Microenvironment of Chronic Wasting Disease-Infected Reeves' Muntjac Deer.J Virol. 2017 Jul 12;91(15):e00501-17. doi: 10.1128/JVI.00501-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28539446 Free PMC article.
-
Current evidence on the transmissibility of chronic wasting disease prions to humans-A systematic review.Transbound Emerg Dis. 2018 Feb;65(1):37-49. doi: 10.1111/tbed.12612. Epub 2017 Jan 30. Transbound Emerg Dis. 2018. PMID: 28139079
-
Differences between the white-tailed and mule deer chronic wasting disease agents after passage through sheep.Front Vet Sci. 2025 Jul 22;12:1632936. doi: 10.3389/fvets.2025.1632936. eCollection 2025. Front Vet Sci. 2025. PMID: 40765750 Free PMC article.
-
Chronic wasting disease of cervids.Curr Top Microbiol Immunol. 2004;284:193-214. doi: 10.1007/978-3-662-08441-0_8. Curr Top Microbiol Immunol. 2004. PMID: 15148993 Review.
References
-
- https://www.usgs.gov/media/images/distribution-chronic-wasting-disease-n.... Distribution of Chronic Wasting Disease [USGS] (National Wildlife Health Center, 2024).
-
- Sohn, H. J. et al. A case of chronic wasting disease in an elk imported to Korea from Canada. J. Vet. Med. Sci.64, 855–858. 10.1292/jvms.64.855 (2002). - PubMed
-
- Williams, E. S. & Young, S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J. Wildl. Dis.16, 89–98 (1980). - PubMed
-
- Williams, E. S. & Miller, M. W. Chronic wasting disease in deer and elk in North America. Rev. Sci. Tech.21, 305–316. 10.20506/rst.21.2.1340 (2002). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

