Virtual brain twins for stimulation in epilepsy
- PMID: 40764764
- PMCID: PMC12457187
- DOI: 10.1038/s43588-025-00841-6
Virtual brain twins for stimulation in epilepsy
Abstract
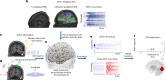
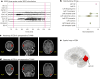
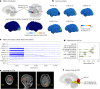
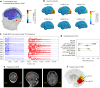
Estimating the epileptogenic zone network (EZN) is an important part of the diagnosis of drug-resistant focal epilepsy and has a pivotal role in treatment and intervention. Virtual brain twins provide a modeling method for personalized diagnosis and treatment. They integrate patient-specific brain topography with structural connectivity from anatomical neuroimaging such as magnetic resonance imaging, and dynamic activity from functional recordings such as electroencephalography (EEG) and stereo-EEG (SEEG). Seizures show rich spatial and temporal features in functional recordings, which can be exploited to estimate the EZN. Stimulation-induced seizures can provide important and complementary information. Here we consider invasive SEEG stimulation and non-invasive temporal interference stimulation as a complementary approach. This paper offers a high-resolution virtual brain twin framework for EZN diagnosis based on stimulation-induced seizures. It provides an important methodological and conceptual basis to make the transition from invasive to non-invasive diagnosis and treatment of drug-resistant focal epilepsy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: V.J., B.D., P.T., H.E.W. and A.W. hold a patent related to the technology and methods discussed in this article: A method and system for estimating an epileptogenic zone network: European Patent EP23169009.0.
Figures



References
-
- Bartolomei, F. et al. Defining epileptogenic networks: contribution of SEEG and signal analysis. Epilepsia58, 1131–1147 (2017). - PubMed
-
- Isnard, J. et al. French guidelines on stereoelectroencephalography (SEEG). Neurophysiol. Clin.48, 5–13 (2018). - PubMed
-
- Bartolomei, F., Chauvel, P. & Wendling, F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain131, 1818–1830 (2008). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

