The role of SMARCA4 in lung cancer
- PMID: 40764807
- PMCID: PMC12326009
- DOI: 10.1038/s41598-025-13913-4
The role of SMARCA4 in lung cancer
Abstract
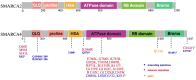
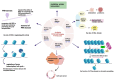
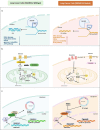
Lung cancer remains one of the leading causes of mortality among cancer patients. Chromatin remodeling is a crucial epigenetic process in cancer, primarily regulated by alterations in genes encoding subunits of the switch/sucrose non-fermentable (SWI/SNF) complex. SMARCA4 and SMARCA2 are two mutually exclusive catalytic subunits within the SWI/SNF complex responsible for utilizing ATP hydrolysis to provide energy for chromatin remodeling. In lung cancer, SMARCA4 is one of the most common mutated subunits of the SWI/SNF complex. Patients with SMARCA4-deficient lung cancer typically experience poorer clinical outcomes. At present, there is no established targeted therapy designed specifically for this type of cancer. Here, we mainly discuss the mechanisms of SMARCA4 in lung cancer development and progression, its co-mutations and mutually exclusive mutations, cellular origin, clinical pathological characteristics, diagnosis and treatment of SMARCA4-deficient lung cancer. We also investigate the concept of synthetic lethality between SMARCA4 and SMARCA2, along with susceptibility to SMARCA4-deficient lung cancer and potential applications of immunotherapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Case Report: FAP+ fibroblasts and SPP1+ macrophages in SMARCA2-deficient while SMARCA4-preserved poorly differentiated lung adenocarcinoma: two case reports and multi-omics analysis.Front Immunol. 2025 May 16;16:1568556. doi: 10.3389/fimmu.2025.1568556. eCollection 2025. Front Immunol. 2025. PMID: 40453096 Free PMC article.
-
Loss of SMARCA4 Leads to Intron Retention and Generation of Tumor-Associated Antigens in Small Cell Carcinoma of the Ovary, Hypercalcemic Type.Cancer Res. 2025 Jul 15;85(14):2626-2642. doi: 10.1158/0008-5472.CAN-24-0044. Cancer Res. 2025. PMID: 40407709 Free PMC article.
-
SMARCA4 controls state plasticity in small cell lung cancer through regulation of neuroendocrine transcription factors and REST splicing.J Hematol Oncol. 2024 Jul 30;17(1):58. doi: 10.1186/s13045-024-01572-3. J Hematol Oncol. 2024. PMID: 39080761 Free PMC article.
-
Genetic drivers of tumor microenvironment and immunotherapy resistance in non-small cell lung cancer: the role of KEAP1, SMARCA4, and PTEN mutations.J Immunother Cancer. 2025 Aug 5;13(8):e012288. doi: 10.1136/jitc-2025-012288. J Immunother Cancer. 2025. PMID: 40764107 Free PMC article. Review.
-
Atypical Site of Presentation of a Rare Type of SMARCA4-Positive Cutaneous Squamous Cell Carcinoma of the Skin: Case Report and Review of the Literature.J Investig Med High Impact Case Rep. 2024 Jan-Dec;12:23247096241271977. doi: 10.1177/23247096241271977. J Investig Med High Impact Case Rep. 2024. PMID: 39215660 Free PMC article. Review.
References
-
- Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229-263 (2024). - PubMed
-
- Arnaud, O., Le Loarer, F. & Tirode, F. BAFfling pathologies: alterations of BAF complexes in cancer. Cancer Lett.419, 266–279 (2018). - PubMed
-
- Alessi, J. V. et al. SMARCA4 and other switch/sucrose nonfermentable family genomic alterations in NSCLC: clinicopathologic characteristics and outcomes to immune checkpoint Inhibition. J. Thorac. Oncol.16, 1176–1187 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

