Doppler ultrasound and 2D shear wave ultrasound elastography for liver fibrosis evaluation in Fontan-associated liver disease
- PMID: 40764907
- PMCID: PMC12323099
- DOI: 10.1186/s12876-025-04151-z
Doppler ultrasound and 2D shear wave ultrasound elastography for liver fibrosis evaluation in Fontan-associated liver disease
Abstract
Background: The Fontan operation improves survival in patients with single ventricle physiology but is associated with Fontan-associated liver disease (FALD), characterized by progressive fibrosis due to prolonged elevated central venous pressure. While 2D shear wave elastography (2D-SWE) can assess fibrosis, it often overestimates stiffness in congestive conditions. Doppler ultrasound, which evaluates hepatic hemodynamics, may complement 2D-SWE for fibrosis assessment. This study evaluated the diagnostic performance of Doppler ultrasound and 2D-SWE in assessing hepatic fibrosis in Fontan patients and compared the findings with biopsy-proven fibrosis severity.
Method: A retrospective study was conducted on 27 Fontan patients who underwent Doppler ultrasound, 2D-SWE, and liver biopsy between January 2020 and December 2022. ROC curves and AUC values were used to assess diagnostic performance.
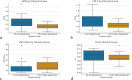
Results: AST to Platelet Ratio Index (APRI) and Fibrosis-4 index (FIB-4) scores demonstrated good discriminatory performance, with AUC values of 0.79 and 0.72, respectively. Resistive Index (RI) of hepatic artery showed moderate discriminatory performance (AUC = 0.62), while 2D-SWE demonstrated poor discriminatory ability (AUC = 0.35). When RI was combined with APRI, the AUC improved from 0.79 to 0.82.
Conclusion: APRI and FIB-4 provided the most accurate assessment of significant fibrosis, while RI of hepatic artery may serve as a useful adjunct to serum biomarkers. Incorporating Doppler ultrasound into a multi-parametric model may improve fibrosis evaluation in Fontan patients.
Keywords: 2D shear wave elastography; Doppler ultrasound; Fontan-associated liver disease; Liver fibrosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This retrospective study was approved by the Institutional Review Board of Chiang Mai University (Study Code: RAD-2566-0229, EC certificate No. 253/2023, approval date: 17 July 2023). The requirement for informed consent was waived due to the retrospective nature of the study, in accordance with institutional policy and national guidelines. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

