Mitigating organophosphate nerve agent, soman (GD), induced long-term neurotoxicity: Saracatinib, a Src Tyrosine Kinase inhibitor, as a potential countermeasure
- PMID: 40764938
- PMCID: PMC12326679
- DOI: 10.1186/s12974-025-03520-5
Mitigating organophosphate nerve agent, soman (GD), induced long-term neurotoxicity: Saracatinib, a Src Tyrosine Kinase inhibitor, as a potential countermeasure
Abstract
Background: Acute exposure to soman (GD), an organophosphate nerve agent (OPNA), irreversibly inhibits acetylcholinesterase (AChE), induces seizures, and could be fatal if not treated immediately. Existing medical countermeasures (MCMs- atropine, oximes, and benzodiazepines) mitigate the acute life-threatening cholinergic symptoms but have limited protection against long-term neurological consequences in survivors. This indicates a need for an effective adjunct therapy to mitigate cognitive, behavioral, and brain pathology associated with OPNA exposure. Saracatinib (SAR), a selective Src tyrosine kinase inhibitor, has emerged as a potential candidate, given its protective properties in experimental models of excitotoxicity and neuroinflammation. Here, we evaluate the therapeutic efficacy of SAR in mitigating long-term neurological deficits triggered by acute exposure to soman in a rat model.
Methods: Mixed-sex adult Sprague Dawley rats were exposed to soman (132 μg/kg, s.c.) and immediately treated with atropine (2 mg/kg, i.m.) and HI-6 (125 mg/kg, i.m.). Seizure severity was quantified for an hour before administering midazolam (3 mg/kg, i.m.). One-hour post-midazolam, SAR/vehicle was administered orally for a week and in the diet for 17 weeks. After behavioral testing, brain MRI, and EEG acquisition, animals were perfused with 4% paraformaldehyde 18 weeks post-soman. Serum and cerebrospinal fluid were collected for nitrooxidative markers and proinflammatory cytokine. Brains were processed for neuroinflammation and neurodegeneration markers.
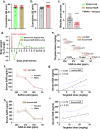
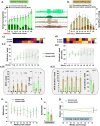
Results: SAR treatment attenuated the soman-induced anxiety/fear-like behavioral changes and motor impairment and modulated the severity of spontaneous seizures. Despite improved hippocampal functional connectivity (fMRI), SAR did not mitigate soman-induced cognitive deficits at 5-7 weeks. However, 18 weeks of SAR treatment demonstrated anti-inflammatory and antioxidant properties, mitigated reactive gliosis and neurodegeneration, and protected somatostatin inhibitory neurons. The glial scars in the amygdala were reduced in SAR-treated animals compared to the vehicle-treated group.
Conclusions: Long-term SAR treatment revealed disease-modifying effects by protecting the brain from soman induced neuroinflammation and neurodegeneration, while also reducing severity of spontaneous seizures. Furthermore, SAR mitigated some soman induced behavioral impairments and brain MRI. These findings highlight the therapeutic potential of Src tyrosine kinase inhibition in soman-induced chronic neurotoxicity.
Keywords: Epilepsy; Glial scar; Neurobehavior; Neuroinflammation; Neurotoxicity; Nitro-oxidative stress; Organophosphate nerve agents; Saracatinib; Soman (GD).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experiments were conducted per the approved protocols by the Institutional Animal Care and Use Committees (IACUC protocol: 23–114) and complied with the NIH ARRIVE Guidelines for the Care and Use of Laboratory Animals. Consent for publication: All authors agreed to publish. Competing interests: The authors declare no competing interests.
Figures










Update of
-
Mitigating Organophosphate Nerve Agent, Soman (GD)-Induced Long-Term Neurotoxicity: Saracatinib, a Src Tyrosine Kinase Inhibitor, as a Potential Countermeasure.Res Sq [Preprint]. 2025 Jun 24:rs.3.rs-6674766. doi: 10.21203/rs.3.rs-6674766/v1. Res Sq. 2025. Update in: J Neuroinflammation. 2025 Aug 5;22(1):199. doi: 10.1186/s12974-025-03520-5. PMID: 40678225 Free PMC article. Updated. Preprint.
References
-
- Apland J, Aroniadou-Anderjaska V, Figueiredo T, Green C, Swezey R, Yang C, Qashu F, Braga M. Efficacy of the GluK1/AMPA receptor antagonist LY293558 against seizures and neuropathology in a soman-exposure model without pretreatment and its pharmacokinetics after intramuscular administration. J Pharmacol Exp Ther. 2013;344(1):133–40. 10.1124/jpet.112.198689. - PMC - PubMed
-
- Aroniadou-Anderjaska V, Apland J, Figueiredo T, Furtado M, Braga M Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: History, mechanisms of action, and medical countermeasures. NEUROPHARMACOLOGY 2020;181. 10.1016/j.neuropharm.2020.108298. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

