Decreased IL-33 in the brain following repetitive mild traumatic brain injury contributes to cognitive impairment by inhibiting microglial phagocytosis
- PMID: 40764944
- PMCID: PMC12323175
- DOI: 10.1186/s40779-025-00631-1
Decreased IL-33 in the brain following repetitive mild traumatic brain injury contributes to cognitive impairment by inhibiting microglial phagocytosis
Abstract
Background: Repetitive mild traumatic brain injury (rmTBI) is a significant risk factor for neurodegeneration, characterized by pathological protein deposition and persistent neuroinflammation. Research has observed increased interleukin-33 (IL-33) levels in the peripheral blood of patients with rmTBI, suggesting IL-33 may participate in regulating the pathological development of rmTBI. The study aims to elucidate the impact and mechanism of IL-33 in the progression of neuropathology following rmTBI, and to explore its potential as a therapeutic target to improve the neurological outcome.
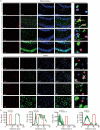
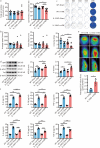
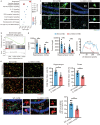
Methods: The study employed an rmTBI mouse model using the wild-type (WT) and IL-33 knockout mice. Cognitive function was assessed via the Y-maze and Barnes tests. The main cell type expressing IL-33 and its receptor, suppression of tumorigenicity 2 (ST2), was then investigated in the mouse brain through immunofluorescence colocalization. As the primary neural cell responsible for ST2 expression, microglia were studied in vitro using the BV2 cell line. The effects of lipid droplets (LDs) accumulation and amyloid-beta (Aβ) phagocytosis were measured to elucidate the impact of IL-33 on BV2 cells' phagocytosis. Additionally, HT22 neuronal apoptosis was assessed by flow cytometry. Finally, the cognitive effects of intranasal administration of IL-33 were evaluated in mice.
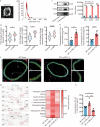
Results: IL-33KO mice exhibited pronounced cognitive impairment after rmTBI. In the mouse brain, astrocytes were identified as the primary source of IL-33 secretion, while microglia predominantly expressed ST2. Transcriptome sequencing revealed that IL-33 significantly influenced phagocytosis function. IL-33 mitigated LDs accumulation in BV2 cells and enhanced Aβ phagocytosis in vitro. In addition, the culture medium of BV2 cells with activated IL-33/ST2 signaling reduced HT22 neuronal apoptosis and axonal damage. Furthermore, intranasal administration of IL-33 was observed to be effective in alleviating neurodegeneration and cognitive outcome of rmTBI mice.
Conclusions: Dysfunction of the IL-33/ST2 axis following rmTBI leads to cognitive dysfunction via impairing microglial phagocytosis capacity and promoting neuronal damage. IL-33 would be a promising therapeutic target for alleviating neurodegeneration following rmTBI.
Keywords: Cognition; Interleukin-33 (IL-33); Microglia; Repetitive mild traumatic brain injury (rmTBI).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The collection and processing of clinical samples for this study were approved by the Ethics Committee of the General Hospital of Tianjin Medical University (IRB2021-YX-056–01), and informed consent was obtained from all participants at enrollment. All animal studies were approved by the Animal Care and Use Committee at Tianjin Medical University (IRB2024-DWFL-044) and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Jiang JY, Gao GY, Feng JF, Mao Q, Chen LG, Yang XF, et al. Traumatic brain injury in China. Lancet Neurol. 2019;18(3):286–95. - PubMed
-
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. - PubMed
-
- Armstrong RC, Sullivan GM, Perl DP, Rosarda JD, Radomski KL. White matter damage and degeneration in traumatic brain injury. Trends Neurosci. 2024;47(9):677–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

