Ethical and regulatory requirements for conducting researcher-driven large-scale multinational genetic haematological studies: the INHERENT experience
- PMID: 40765057
- PMCID: PMC12323221
- DOI: 10.1186/s12961-025-01375-z
Ethical and regulatory requirements for conducting researcher-driven large-scale multinational genetic haematological studies: the INHERENT experience
Abstract
Background: The International Hemoglobinopathy Research Network (INHERENT) focuses on studying genetic modifiers through large, multi-ethnic genome-wide association studies involving paediatric and adult patients with haemoglobinopathies. The growing integration of genetics and genomics into global healthcare has highlighted the need for standardized policies on biospecimen and data handling. This study describes the necessary ethical and regulatory framework for conducting multinational, researcher-driven genetic studies on humans.
Methods: Key areas related to the INHERENT study were identified through collaborative research. A review of the grey literature was performed, consulting official sources. An online survey was conducted to identify the local rules.
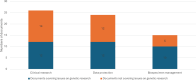
Results: Despite the availability of 33 international documents applicable to the three key areas of our investigation, i.e. personal data processing, clinical research and biospecimen management, there is no unique reference for genetic studies without investigational drugs, i.e. outside the scope of good clinical practice. Specific laws and guidelines/recommendations governing the processing of personal data and privacy have been released in most of the 32 surveyed countries. As an example, discordances were found regarding the requirement to get approval from the ethics committees.
Conclusions: Such heterogeneity challenges the scientific community in conducting these genetic studies. This study calls for further efforts to harmonize international standards for genetic research.
Keywords: Ethics; Genetics; Genomics; Harmonization; Hemoglobinopathies; Multicentre study; Regulatory; Researcher-driven study; Standards.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Uffelmann E, Huang QQ, Munung NS, De Vries J, Okada Y, Martin AR, et al. Genome-wide association studies. Nat Rev Methods Primers. 2021;1(1):59.
-
- Kountouris P. INHERENT-International Hemoglobinopathy Research Network. Presented at the 17th Annual Sickle Cell and Thalassaemia Conference (ASCAT). In London; 2022.
-
- NIH- National Library of Medicine_Clinicaltrials.gov. Study of the role of genetic modifiers in hemoglobinopathies (INHERENT). Available from: https://clinicaltrials.gov/study/NCT05799118
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

