Inhibition of Mettl3 alleviates low-dose cisplatin-induced renal fibrosis and enhances the chemotherapeutic efficacy in mouse models of cancer
- PMID: 40765834
- PMCID: PMC12320029
- DOI: 10.7150/ijbs.117443
Inhibition of Mettl3 alleviates low-dose cisplatin-induced renal fibrosis and enhances the chemotherapeutic efficacy in mouse models of cancer
Abstract
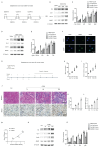
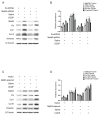
Cisplatin (CDDP), a commonly utilized anti-tumor drug, leads to acute kidney injury (AKI) and chronic kidney disease (CKD). The mechanisms and therapeutic approaches for injury in AKI have been extensively studied, but the mechanisms resulting in CKD are poorly comprehended and intervention methods are scarce. In the current study, we found that under different phases of the repeated low-dose CDDP treatment, Mettl3 expression was induced by two different mechanisms. In the presence of CDDP, the transcription factor Hif1-α was induced, resulting in an increase in Mettl3. When CDDP was removed, the previously increased Mettl3 caused an elevated lactate level, which formed a positive feedback loop by mutually reinforcing each other's expression via H3K18 lactylation. Functionally, we disclose that the knockout of Mettl3 in proximal tubules mitigates repeated low-dose CDDP-induced renal fibrosis both in vitro and in vivo. Mechanistically, Mettl3 stabilizes Pfkfb3 mRNA through N6-methyladenosine (m6A) modification and subsequently induces lactate production to upregulate the PD-L1 expression via H3K18 lactylation, thereby promoting both tumor growth and CDDP-induced renal damage. Intriguingly, we discovered that Levosimendan suppresses the methyltransferase activity of Mettl3 to lower the m6A level but has no impact on the abundance of the Mettl3-Mettl14 complex. PLGA-encapsulated Levosimendan not only alleviates repeated low-dose CDDP-induced renal fibrosis, but also significantly enhances the chemotherapeutic effects of cisplatin in several xenograft and syngeneic mouse tumor models by suppressing the Mettl3/Pfkfb3/lactate/ H3K18la/PD-L1 axis. Collectively, targeting Mettl3 might offer an effective therapeutic strategy during cisplatin-based chemotherapy-induced renal fibrosis, and PLGA-encapsulated Levosimendan is a potential intervention approach.
Keywords: Mettl3; acute kidney injury; renal fibrosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







References
-
- Tang C, Livingston MJ, Safirstein R, Dong Z. Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat Rev Nephrol. 2023;19:53–72. - PubMed
-
- Su H, Ye C, Lei CT, Tang H, Zeng JY, Yi F. et al. Subcellular trafficking of tubular MDM2 implicates in acute kidney injury to chronic kidney disease transition during multiple low-dose cisplatin exposure. Faseb j. 2020;34:1620–36. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

