Analysis of control arms in oncology randomised trials in Brazil: a cross-sectional study
- PMID: 40765840
- PMCID: PMC12323517
- DOI: 10.1136/bmjonc-2025-000808
Analysis of control arms in oncology randomised trials in Brazil: a cross-sectional study
Abstract
Objective: Low-middle-income countries (LMICs) have increased their participation in international oncology trials. However, considerable disparities in treatment standards across countries have raised ethical concerns regarding the use of control arms that may not align with the established standards of care in high-income countries. This trial aims to describe the control arms of randomised oncology trials recruiting in Brazil, an LMIC where the majority of patients receive care through the public health system, the Unified Health System (SUS), which provides limited access to cancer treatments.
Methods and analysis: This cross-sectional study included randomised clinical trials recruiting in Brazil on 4 December 2023 (ClinicalTrials.gov). Abstracted data included sample size, sponsor, tumour site, study phase and control arm. Two independent investigators classified control arms as superior, equal or inferior based on National Comprehensive Cancer Network (NCCN), Brazilian private insurance and SUS standards. Data were summarised in means, medians and proportions. Fisher's exact test compared categories. A p<0.05 was considered statistically significant.
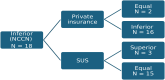
Results: A total of 98 studies were included. The median intended sample size was 555 patients (54-6000). Most studies were phase 3 (84.7%) and pharma-sponsored (97%). Lung (29.6%) and breast (24.4%) were the most commonly studied tumour sites. Regarding treatment setting, 23 studies (23.5%) were (neo)adjuvant trials, 48 (49.0%) first-line and 27 (27.5%) second-line or later. Overall, 80 (81.7%), 82 (83.7%) and 58 studies (59.1%) employed control arms considered equivalent to the standards of NCCN, private insurance and SUS, respectively. 18 studies (18.3%) had a suboptimal control arm according to NCCN guidelines, while 16 studies (16.3%) according to Brazilian private insurance. No studies used control arms inferior to SUS standards. Of the 18 control arms inferior to NCCN, 3 were superior and 15 were equal to standard of care offered by SUS. No studies had their control arms superior to NCCN or private insurance; whereas, 40 (40.9%) were superior to SUS.
Conclusion: A significant number of studies employed control arms inferior to NCCN guidelines; however, these were considered superior or equal to the standards offered by SUS. Such discrepancies may hinder the appropriate interpretation of study findings.
Keywords: Clinical Trial; Ethics; Neoplasms.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Daniel Vilarim Araujo received honoraria from: MSD, Libbs, AstraZeneca, Novartis and Tersera. Provided consulting services to MSD Oncology.
Figures
Comment in
-
Inferior control arms in oncology trials in LMICs: contextualised or compromised?BMJ Oncol. 2025 Aug 25;4(1):e000931. doi: 10.1136/bmjonc-2025-000931. eCollection 2025. BMJ Oncol. 2025. PMID: 40909186 Free PMC article. No abstract available.
References
-
- Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49:509.:509. - PubMed
-
- Geneva: World Health Organization; 2018. Pricing of cancer medicines and its impacts.https://iris.who.int/handle/10665/277190 Available.
LinkOut - more resources
Full Text Sources