Cardiovascular risk factors in myeloproliferative neoplasms: associations with survival and thrombotic outcomes
- PMID: 40765906
- PMCID: PMC12320413
- DOI: 10.1016/j.bvth.2025.100051
Cardiovascular risk factors in myeloproliferative neoplasms: associations with survival and thrombotic outcomes
Abstract
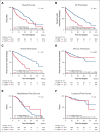
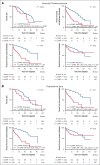
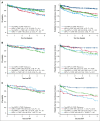
Cardiovascular risk factors (CVRFs) are important modifiers of thrombosis in patients with essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF). We performed a retrospective cohort analysis evaluating CVRFs in 1005 patients with myeloproliferative neoplasms (MPNs) from the Dana-Farber Cancer Institute Hematologic Malignancies Data Repository from 2014 to 2023. We also included a non-MPN group of 1543 age- and sex-matched controls with no known diagnoses of hematologic malignancies to evaluate whether CVRFs differentially affected outcomes. CVRFs were identified through International Classification of Diseases codes for hypertension, hyperlipidemia, type 2 diabetes mellitus (DM2), current smoking, or body mass index ≥30 before MPN diagnosis. CVRFs occurred in 34% of patients with MPNs. Patients with MPN with ≥1 CVRF had increased risk of death (hazard ratio [HR], 2.52; 95% confidence interval [CI], 1.9-3.35) and arterial/venous thrombosis (HR, 3.05; 95% CI 2.39-3.92). Within MPN subtypes, patients with ET, PV, and MF who had CVRFs also demonstrated worse overall survival and thrombotic outcomes. Among CVRFs, only DM2 predicted worse thrombotic outcomes in patients with MPNs. The HR of CVRF on thrombosis was decreased in patients with MPNs compared with non-MPN controls (HR, 0.51; 95% CI, 0.36-0.86). Looking at ET, PV, and MF specifically, the presence of a CVRF also had less of an impact on thrombotic risk in ET compared with controls (HR, 0.35; P = .019); no interactions between MPN diagnosis and CVRFs were seen in patients with PV and MF. Our results underscore both the necessity of managing CVRFs in MPNs to improve patient morbidity and mortality and the need to ameliorate thrombotic risk with measures beyond addressing CVRFs.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.H. reports consultancy fees from PharmaEssentia and Merck. L.D.W. reports consultancy fees from AbbVie and Vertex; and membership on an entity's board of directors or advisory committees in Sobi. M.S. served on the advisory board for Novartis, Kymera, Sierra Oncology, GlaxoSmithKline, Rigel, Bristol Myers Squibb (BMS), Sobi, and Syndax; consulted for Boston Consulting and The Dedham Group; and participated in Continuing Medical Education activity for Novartis, Curis Oncology, Haymarket Media, and Clinical Care Options. D.J.D. reports honoraria from Pfizer, Incyte, Amgen, Takeda, Servier, Jazz, Kite, Blueprint, Autolus, Novartis, and Gilead; and research funding from AbbVie, Novartis, Blueprint, and GlycoMimetics. C.L. reports consultancy fees from Takeda Pharmaceuticals, bluebird bio, Qiagen, Sarepta Therapeutics, Verve Therapeutics, Jazz Pharmaceuticals, and Vertex Pharmaceuticals; and membership on an entity's board of directors or advisory committees in bluebird bio. M.L. reports research funding from AbbVie and Novartis; and honoraria from Jazz, Pfizer, Novartis, and Kite. A.E.-J. reports consulting fees from Incyte, Tuesday Health, Novartis, and GlaxoSmithKline. G.H. reports research funding from Incyte and MorphoSys; membership on an entity's board of directors or advisory committees in Keros, Pharmaxis, Pfizer, MorphoSys, Novartis, Protagonist, AbbVie, and BMS; and is a current holder of stock options in a privately held company in Regeneron. The remaining authors declare no competing financial interests.
Figures







References
-
- Hultcrantz M, Björkholm M, Landgren O, Kristinsson SY, Andersson TML. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018;169(4):268. - PubMed
-
- Hultcrantz M, Wilkes SR, Kristinsson SY, et al. Risk and cause of death in patients diagnosed with myeloproliferative neoplasms in Sweden between 1973 and 2005: a population-based study. J Clin Oncol. 2015;33(20):2288–2295. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
