Elevated tissue factor pathway inhibitor delays thrombin generation in COVID-19 but is not associated with clinical outcomes
- PMID: 40765912
- PMCID: PMC12320461
- DOI: 10.1016/j.bvth.2025.100071
Elevated tissue factor pathway inhibitor delays thrombin generation in COVID-19 but is not associated with clinical outcomes
Abstract
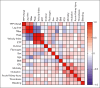
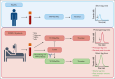
Plasma levels of tissue factor pathway inhibitor (TFPI) are elevated in many patients with COVID-19 but the role of TFPI in COVID-19 coagulopathy remains elusive. We sought to determine the contribution of TFPI to thrombin generation in patients with COVID-19 and assess its association with thrombosis and other clinical outcomes. We used blood samples from an early COVID-19 clinical trial of adult patients hospitalized with acute COVID-19 from April 2020 to January 2021 (ClinicalTrials.gov identifier: NCT04360824). Plasma TFPI was measured by enzyme-linked immunosorbent assay, and thrombin generation potential was measured in the presence or absence of TFPI neutralizing antibodies. Thromboelastography was performed with whole-blood samples. We found that plasma TFPI was elevated in patients with COVID-19 compared with healthy individuals. Thrombin generation triggered by exogenous TF and phospholipids was increased in COVID-19, reflected by greater peak thrombin, velocity index, and endogenous thrombin potential; however, the time to initiation of thrombin generation (lag time) was delayed. Addition of a neutralizing anti-TFPI antibody significantly shortened the lag time in COVID-19 and normalized the difference in lag time between those with COVID-19 and healthy individuals. Plasma TFPI was positively associated with lag time, time to peak thrombin, and time to initial clot formation in thromboelastography. Multivariate analysis demonstrated that TFPI correlated with lag time and time to reach peak thrombin but not with 30-day mortality, thrombosis, or other adverse clinical outcomes. We conclude that elevated plasma TFPI delays the initiation of thrombin generation and clot formation but is not associated with thrombosis in patients hospitalized with COVID-19.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





Similar articles
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Examining downstream effects of concizumab in hemophilia A with a mathematical modeling approach.J Thromb Haemost. 2025 Feb;23(2):480-491. doi: 10.1016/j.jtha.2024.10.028. Epub 2024 Nov 12. J Thromb Haemost. 2025. PMID: 39536817 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age.Cochrane Database Syst Rev. 2014 May 22;2014(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. Cochrane Database Syst Rev. 2014. PMID: 24852077 Free PMC article.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
