Fibroblast Growth Factor 21 Promotes Vascular Smooth Muscle Cell Contractile Polarization via p38 Mitogen-Activated Protein Kinase-Promoted Serum Response Factor Phosphorylation
- PMID: 40765997
- PMCID: PMC12324144
- DOI: 10.34133/research.0815
Fibroblast Growth Factor 21 Promotes Vascular Smooth Muscle Cell Contractile Polarization via p38 Mitogen-Activated Protein Kinase-Promoted Serum Response Factor Phosphorylation
Abstract
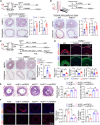
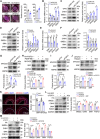
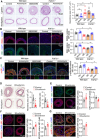
Phenotypic abnormalities in vascular smooth muscle cells (VSMCs) are believed to play essential roles in the progression of vascular diseases. Here, we explored the impact of fibroblast growth factor 21 (FGF21) on the phenotypic transition of VSMCs. Our findings revealed that FGF21 expression was substantially down-regulated in both human and mouse neointimal regions. Additionally, plasma FGF21 levels were lower in patients with atherosclerotic coronary artery disease (ASCAD) compared to those without ASCAD. Similarly, patients with restenosis exhibited reduced FGF21 levels compared to those without restenosis. In vivo, FGF21 deficiency accelerated intimal hyperplasia and decreased the number of contractile VSMCs in mouse neointima. However, hepatocyte-specific FGF21 knockout had no effect on ligation-induced intimal hyperplasia. Conversely, administration of recombinant FGF21 protein reduced neointima formation. This effect was abolished in mice with β-klotho VSMC-specific knockout, suggesting a direct effect of FGF21 on VSMCs. In vitro, FGF21 could promote the contractile phenotype transition of human aortic smooth muscle cells under basal or platelet-derived growth factor-BB incubation conditions. Furthermore, FGF21 activation led to the phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK), which subsequently formed a complex with the serum response factor (SRF)-myocardin complex. This complex increased the phosphorylation of SRF at serine 224, thereby enhancing the transcription activation of the SRF-myocardin complex. Finally, we revealed that treatment with the FGF21 analog efruxifermin or activation of p38 MAPK using anisomycin effectively inhibited neointima formation. Taken together, these results indicate that modulating FGF21 or its subsequent signal pathways could serve as a therapeutic strategy for vascular diseases characterized by abnormal VSMC phenotypic transition.
Copyright © 2025 Mengmeng Zhu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Sahin B, Ilgun G. Risk factors of deaths related to cardiovascular diseases in World Health Organization (WHO) member countries. Health Soc Care Community. 2022;30(1):73–80. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

