Multi-Faceted Roles of ERCC1-XPF Nuclease in Processing Non-B DNA Structures
- PMID: 40766048
- PMCID: PMC12323751
- DOI: 10.3390/dna2040017
Multi-Faceted Roles of ERCC1-XPF Nuclease in Processing Non-B DNA Structures
Abstract
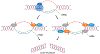
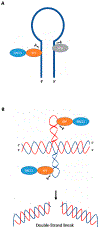
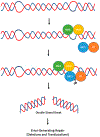
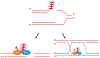
Genetic instability can result from increases in DNA damage and/or alterations in DNA repair proteins and can contribute to disease development. Both exogenous and endogenous sources of DNA damage and/or alterations in DNA structure (e.g., non-B DNA) can impact genome stability. Multiple repair mechanisms exist to counteract DNA damage. One key DNA repair protein complex is ERCC1-XPF, a structure-specific endonuclease that participates in a variety of DNA repair processes. ERCC1-XPF is involved in nucleotide excision repair (NER), repair of DNA interstrand crosslinks (ICLs), and DNA double-strand break (DSB) repair via homologous recombination. In addition, ERCC1-XPF contributes to the processing of various alternative (i.e., non-B) DNA structures. This review will focus on the processing of alternative DNA structures by ERCC1-XPF.
Keywords: DNA repair; ERCC1-XPF; genetic instability; non-B DNA structure.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures
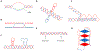

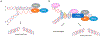
References
-
- Bindra RS; Glazer PM Genetic instability and the tumor microenvironment: Towards the concept of microenvironment-induced mutagenesis. Mutat. Res 2005, 569, 75–85. - PubMed
-
- Beckman RA; Loeb LA Genetic instability in cancer: Theory and experiment. Semin. Cancer Biol 2005, 15, 423–435. - PubMed
-
- Breivik J; Gaudernack G Genomic instability, DNA methylation, and natural selection in colorectal carcinogenesis. Semin. Cancer Biol 1999, 9, 245–254. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
