Specific reaction conditions for efficient automated 68Ga-radiolabeling of the FAP-2286 pseudopeptide on a GAIA® synthesizer
- PMID: 40766056
- PMCID: PMC12321554
- DOI: 10.3389/fmed.2025.1628158
Specific reaction conditions for efficient automated 68Ga-radiolabeling of the FAP-2286 pseudopeptide on a GAIA® synthesizer
Abstract
Introduction: Automated radiolabeling of gallium-68-labeled experimental radiopharmaceuticals is crucial for ensuring high reproducibility and regulatory compliance in clinical settings. FAP-2286, a promising DOTA-pseudopeptide targeting the tumor microenvironment, has demonstrated superior tumor retention compared to quinoline-based analogs, making it an attractive theranostic agent. This study aimed to optimize and automate the preparation of [68Ga]Ga-FAP-2286 on the GAIA® synthesizer, ensuring high radiochemical purity (RCP) and radiochemical yield (RCY).
Methods: Manual radiolabeling assays were initially performed to identify optimal reaction conditions, varying buffer, antioxidant, vector amount, heating time, and purification methods. The selected conditions were then adapted to an automated protocol using a GAIA® module. A strong cation exchange (SCX) cartridge for 68Ga pre-concentration and a solid-phase extraction (SPE) step for final purification were included in the process. RCY, RCP, and stability over 4 h were assessed using radio-HPLC and radio-TLC. Additionally, the applicability of the optimized automated method was evaluated for 3BP-3940, a structurally related pseudopeptide.
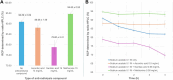
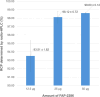
Results: Initial optimization studies identified sodium acetate buffer 0.1 M with methionine as an antioxidant, 25 μg of FAP-2286, and a 4-min heating time as the best manual radiolabeling conditions, achieving a RCP > 98%. In the automated synthesis, adjustments were made, including doubling the vector amount and extending heating to 9 min, resulting over three test-batches in a moderate RCY of 59.85 ± 3.73% and a RCP just over 94% up to 4 h after the end of synthesis. Importantly, the method was successfully transposed to [68Ga]Ga-3BP-3940, yielding better RCY (75.62 ± 11.76%), RCP and stability profiles (> 95.95% over 4 h).
Conclusion: This study established a robust, automated protocol for the synthesis of [68Ga]Ga-FAP-2286, ensuring high purity, reproducibility, and compatibility with clinical applications. The method's successful adaptation to 3BP-3940 highlights its versatility for such radiopharmaceuticals, supporting the broader implementation of automated theranostic agent production in nuclear medicine.
Keywords: FAP-2286; PET imaging; automated radiolabeling; gallium-68; radiopharmaceuticals; tumor microenvironment.
Copyright © 2025 Ammour, Torchio, Renaud, Rubira and Fersing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

![Flowchart of the synthesis process for Gallium-68 ([68Ga]) labeled FAP-2286. The left side lists 15 step-by-step procedures for the synthesis, starting with tubing set preparation and ending with final product vial release. The right side illustrates the setup, including equipment like the generator, SCX and SPE cartridges, reaction medium, and final product vial. Key stages include reaction medium preparation and washing processes, detailing specific conditions like temperatures and solution concentrations.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/971d/12321554/6704e1e85c97/fmed-12-1628158-g003.gif)




References
LinkOut - more resources
Full Text Sources
Miscellaneous

