A stable systemic infection of methicillin-resistant Staphylococcus aureus (MRSA) in cynomolgus macaques produces extended window for therapeutic intervention
- PMID: 40766078
- PMCID: PMC12322839
- DOI: 10.3389/fmicb.2025.1601381
A stable systemic infection of methicillin-resistant Staphylococcus aureus (MRSA) in cynomolgus macaques produces extended window for therapeutic intervention
Abstract
Introduction: Staphylococcus aureus is a common gram-positive commensal that, upon entering the bloodstream, can cause devastating illness and death within hours or days. Methicillin-resistant S. aureus (MRSA) infections, now a leading cause of bloodstream infections worldwide, pose significant challenges due to their rapid progression, high mortality rates, and limited therapeutic options. While there are prevalent small animal models of experimental MRSA infection, there has been minimal development of larger mammalian models capable of recapitulating clinical aspects of human systemic MRSA infection.
Methods: Following a pilot study to determine the optimal dose and route to establish systemic MRSA infection, we challenged six cynomolgus macaques with 109 colony-forming units MRSA (lineage USA300) via intravenous (IV) route. Animals were monitored closely up to 8 days for physiological, immunological, and cellular endpoints. Histopathology was performed on tissues collected 2 and 8 days after infection.
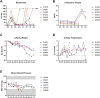
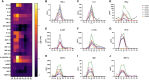
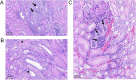
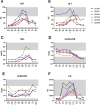
Results: An IV dose of 109 CFU MRSA USA300 in cynomolgus macaques produced bacteremia resulting from multifocal invasive infections, elevated markers of systemic inflammation, as well as weight loss, fever, and hemodynamic changes consistent with bloodstream infection. Hematological analyses demonstrated neutrophilic leukocytosis, lymphocytopenia, monocytosis, and mild thrombocytopenia. We observed a robust cytokine response, including TNF-α, IL-6, G-CSF, and IL-1RA, peaking 6 h post-infection. Flow cytometry immunophenotyping revealed dynamic shifts in circulating monocyte subpopulations, and histopathological analysis demonstrated multi-organ damage with significant findings in the kidneys, heart, liver, and lungs. By 8 days post-infection, moderate to severe myocardial, renal, and hepatic dysfunction were evident, supported by changes in clinical chemistry biomarkers. None of the animals required euthanasia before the scheduled date of termination.
Discussion: In this study, we establish a non-human primate model of systemic MRSA infection that allows for the characterization of MRSA pathogenesis and evaluation of therapeutics over a period of days rather than hours. This model successfully recapitulates key aspects of human MRSA bloodstream infections, providing a valuable platform for evaluating therapeutic interventions and understanding disease mechanisms.
Keywords: MRSA; S. aureus bacteremia; Staphylococcus aureus; bacterial infection model; cynomolgus macaque; nonhuman primate; sepsis.
Copyright © 2025 Gamblin, Peterson, Simon, Orekov, Penn, Shumyatsky, Patel, Womer, Kabir, Bushby, Muench, Feliciano, Paz, Finneyfrock, Cook and Kar.
Conflict of interest statement
AG, SS, MP, TO, EP, GS, GP, AW, HK, DB, JM, KF, FP, BF, AC, and SK were employed by the company BIOQUAL, Inc.
Figures





References
-
- Baddour L., Wilson W., Bayer A., Fowler V., Tleyjeh I., Rybak M., et al. (2015). Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 132 1435–1486. 10.1161/CIR.0000000000000296 - DOI - PubMed
-
- Bain P., Latimer K. (2011). “Chapter 7. Liver,” in Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 5th Edn, eds Kenneth S., Latimer (Chichester: Wiley-Blackwell; ), 213–214.
-
- Bandaru S., Killeen R., Gupta V. (2025). Poikilocytosis. Treasure Island, FL: StatPearls Publishing. - PubMed
LinkOut - more resources
Full Text Sources

