Anti-IgE therapy versus allergen-specific immunotherapy for food allergy: weighing the pros and cons
- PMID: 40766311
- PMCID: PMC12321543
- DOI: 10.3389/fimmu.2025.1617153
Anti-IgE therapy versus allergen-specific immunotherapy for food allergy: weighing the pros and cons
Abstract
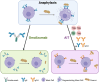
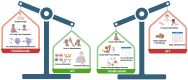
With the recent FDA approval of the anti-IgE biologic, omalizumab, in 2024 for the treatment of food allergy, it is critical to consider the advantages and disadvantages of anti-IgE and allergen-specific immunotherapies (AITs) to help determine optimal patient care. Several AITs have been studied for food allergy, including oral (OIT), sublingual (SLIT), and epicutaneous immunotherapy (EPIT) with varying degrees of safety and efficacy. There are obvious advantages of treating food allergies with omalizumab, including less frequent administration (every 2 or 4 weeks) compared to the daily dosing of AITs, treating multiple food allergies with one medication, and the potential benefit for comorbid asthma and environmental allergies. However, disadvantages of omalizumab include the requirement for lifelong treatment of a costly biologic that will not induce immunologic tolerance. On the other hand, AITs have been shown to effectively induce desensitization in most individuals and can lead to long-term tolerance or remission in a subset of patients. In this review, we will discuss the pros and cons of omalizumab and AITs and the potential benefit of combining both approaches in young children to achieve immediate increases in reaction threshold while also inducing tolerogenic immunologic responses.
Keywords: IgE; allergy immunotherapy; desensitization; epicutaneous immunotherapy; omalizumab; oral immunotherapy; sublingual immunotherapy.
Copyright © 2025 Kulis, Humphrey, Krempski, Kim and Smeekens.
Conflict of interest statement
Author MK reports grant funding from NIH-NIAID and the Department of Defense. Author EK reports consulting fees from ALK, Cellergy Pharma, DBV Technologies, Genentech, Hanimune Therapeutics, IgGenix, Novartis, Phylaxis BioScience, Revolo Biotherapeutics; safety review committee participation for Allergy Therapeutics; and grants to his university from NIH-NIAID, and Food Allergy Research and Education FARE. Author JS reports grant funding from FARE. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

