Рrospective multicenter study of treatment efficacy, safety, and quality of life in a large cohort of patients with inborn errors of immunity receiving subcutaneous immunoglobulin by the rapid push method
- PMID: 40766319
- PMCID: PMC12321879
- DOI: 10.3389/fimmu.2025.1598491
Рrospective multicenter study of treatment efficacy, safety, and quality of life in a large cohort of patients with inborn errors of immunity receiving subcutaneous immunoglobulin by the rapid push method
Abstract
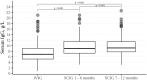
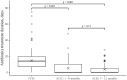
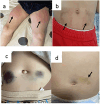
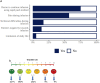
Subcutaneous immunoglobulin (SCIG) preparations are widely used in patients with inborn errors of immunity (IEI), with proven efficacy and good tolerance. We assessed treatment efficacy, safety, and quality of life in a large cohort of IEI patients who switched from intravenous immunoglobulin (IVIG) to SCIG. Our observational study included 200 patients aged 1-65 years with IEI. SCIG Cutaquig (16.5%) was administered every 7-10 days for at least 12 months via the rapid push method. We assessed the rate of infection, immunoglobulin G (IgG) concentration, adverse events, and quality of life. A total of 8,787 SCIG doses were administered during the study. The rate of infections (per person/month) during SCIG treatment was 0.05, which was significantly lower compared to 0.19 during the IVIG period (p<0.001). The median trough IgG was 6.9 g/L on IVIG, compared to 9.0 g/L during the first six months, and 9.2 g/L during the next six months on SCIG. Systemic reactions occurred in 12.4% of the IVIG infusions and 1.9% of the SCIG infusions. The total scores on quality of life summary assessments of physical and mental health were higher on SCIG therapy compared with IVIG (p<0.001). At the end of the study, 85.6% of the patients chose to remain on SCIG. Our data suggest that SCIG infusion via the rapid push method is effective, well tolerated, and feasible in large groups of IEI patients, including those in large countries such as Russia.
Keywords: efficacy; inborn errors of immunity; intravenous immunoglobulin; quality of life; rapid push; safety; subcutaneous immunoglobulin; tolerability.
Copyright © 2025 Avedova, Deripapa, Rodina, Mukhina, Latysheva, Yukhacheva, Burlakov, Kan, Bogdanova, Moiseeva, Kuzmenko, Nesterenko, Deordieva, Ogneva, Bludova, Roppelt, Fomina, Zinovieva, Sevostyanova, Kalmeteva, Prolygina, Barycheva, Selezneva, Shakhova, Laba, Vlasova, Gorenkova, Timofeeva, Trusova, Guseva, Yudina, Grevtseva, Ibisheva, Bambaeva, Mashkovskaya, Isakova, Shakirova, Selina, Shilova, Zubova, Khabaeva, Kitova, Mandzhieva, Starikova, Pavlova, Tyulyakova, Levin, Grachev and Shcherbina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Poli MC, Aksentijevich I, Bousfiha AA, Cunningham-Rundles C, Hambleton S, Klein C, et al. Human inborn errors of immunity: 2024 update on the classification from the International Union of Immunological Societies Expert Committee. J Hum Immun. (2025) 1. doi: 10.70962/jhi.20250003, PMID: - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

