This is a preprint.
Polycomb Repressive-Deubiquitinase Complex Safeguards Oocyte Epigenome and Female Fertility by Restraining Polycomb Activity
- PMID: 40766345
- PMCID: PMC12324195
- DOI: 10.1101/2025.07.24.666633
Polycomb Repressive-Deubiquitinase Complex Safeguards Oocyte Epigenome and Female Fertility by Restraining Polycomb Activity
Abstract
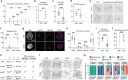
Mouse oocytes exhibit a unique chromatin landscape characterized by broad H3K27ac and H3K27me3 domains, demarcating euchromatin and facultative heterochromatin, respectively. However, the mechanisms underlying this non-canonical landscape remain elusive. Here we report BAP1, a core component of the Polycomb Repressive-Deubiquitinase (PR-DUB) complex, as a key negative regulator of Polycomb activity during oogenesis. BAP1 restricts pervasive H2AK119ub1 accumulation in oocytes and protects oocyte-specific broad H3K27ac, particularly within gene-poor regions, from ectopic H3K27me3 deposition. While PR-DUB has been linked to gene repression, in oocytes BAP1 primarily promotes transcription and contributes minimally to Polycomb-mediated silencing. BAP1-dependent transcriptional activation is essential for oocyte developmental competence and female fertility. BAP1 loss disrupts the maternal-to-zygotic transition and impairs embryonic enhancer activation, ultimately compromising preimplantation development. Notably, while H3K27ac patterns are reset after fertilization, the aberrant H3K27me3 landscape established in BAP1-deficient oocytes persists in early embryos. Together, these findings reveal a critical role for PR-DUB in safeguarding the oocyte epigenome by protecting euchromatin from ectopic Polycomb activity, rather than enforcing transcriptional repression.
Keywords: BAP1; H2AK119ub1; H3K27ac; H3K27me3; PR-DUB; female fertility; oocyte.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures






Similar articles
-
BAP1 enhances Polycomb repression by counteracting widespread H2AK119ub1 deposition and chromatin condensation.Mol Cell. 2021 Sep 2;81(17):3526-3541.e8. doi: 10.1016/j.molcel.2021.06.020. Epub 2021 Jun 28. Mol Cell. 2021. PMID: 34186021 Free PMC article.
-
A mutant ASXL1-BAP1-EHMT complex contributes to heterochromatin dysfunction in clonal hematopoiesis and chronic monomyelocytic leukemia.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2413302121. doi: 10.1073/pnas.2413302121. Epub 2025 Jan 3. Proc Natl Acad Sci U S A. 2025. PMID: 39752521 Free PMC article.
-
H2AK119ub1 guides maternal inheritance and zygotic deposition of H3K27me3 in mouse embryos.Nat Genet. 2021 Apr;53(4):539-550. doi: 10.1038/s41588-021-00820-3. Epub 2021 Apr 5. Nat Genet. 2021. PMID: 33821003
-
Gap junction connexins in female reproductive organs: implications for women's reproductive health.Hum Reprod Update. 2015 May-Jun;21(3):340-52. doi: 10.1093/humupd/dmv007. Epub 2015 Feb 9. Hum Reprod Update. 2015. PMID: 25667189
-
Oocyte activation for women following intracytoplasmic sperm injection (ICSI).Cochrane Database Syst Rev. 2024 Dec 20;12(12):CD014040. doi: 10.1002/14651858.CD014040.pub2. Cochrane Database Syst Rev. 2024. PMID: 39704318
References
-
- Blackledge N.P., Farcas A.M., Kondo T., King H.W., McGouran J.F., Hanssen L.L.P., Ito S., Cooper S., Kondo K., Koseki Y., et al. (2014). Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157, 1445–1459. 10.1016/j.cell.2014.05.004. - DOI - PMC - PubMed
-
- Bonnet J., Boichenko I., Kalb R., Le Jeune M., Maltseva S., Pieropan M., Finkl K., Fierz B., and Muller J. (2022). PR-DUB preserves Polycomb repression by preventing excessive accumulation of H2Aub1, an antagonist of chromatin compaction. Genes Dev 36, 1046–1061. 10.1101/gad.350014.122. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
