This is a preprint.
Antibodies targeting HSV glycoprotein B require effector functions to protect neonatal mice
- PMID: 40766354
- PMCID: PMC12324208
- DOI: 10.1101/2025.07.25.666806
Antibodies targeting HSV glycoprotein B require effector functions to protect neonatal mice
Abstract
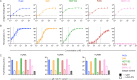
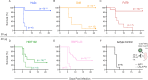
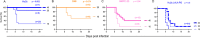
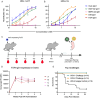
Glycoprotein B (gB) serves as the viral fusion protein for herpes simplex virus (HSV), mediating fusion between viral and host membranes resulting in infection. As such, gB represents a potentially critical target for the host immune system with high potential relevance for vaccine design. Here we investigated the mechanisms of protection for a panel of gB-specific monoclonal antibodies (mAb) in a mouse model of neonatal HSV (nHSV) infection. Viral neutralization contributed, but Fc effector functions were critical for mAb-mediated protection against nHSV mortality, depending on dose. Moreover, AAV-mediated in vivo expression of a gB-specific mAb in mice provided transgenerational protection against HSV-1 and HSV-2 mortality in their offspring. These findings demonstrate that antibodies targeting gB can serve as potent therapeutics and that they require diverse functional profiles to afford optimal protection, informing vaccine design.
Conflict of interest statement
Competing Interests I.M.B., D.A.L., and M.E.A. report a patent, WO2020077119A1, for the use of HSV-specific mAbs as method for the treatment for nHSV infections. M.E.A. reports consulting for Seromyx Systems and research funding from Moderna unrelated to this work. A.B.B. is a founder of Cure Systems LLC.
Figures

Similar articles
-
Effector functions are required for broad and potent protection of neonatal mice with antibodies targeting HSV glycoprotein D.Cell Rep Med. 2024 Feb 20;5(2):101417. doi: 10.1016/j.xcrm.2024.101417. Epub 2024 Feb 12. Cell Rep Med. 2024. PMID: 38350452 Free PMC article.
-
Antibody effector functions are required for broad and potent protection of neonates from herpes simplex virus infection.bioRxiv [Preprint]. 2023 Aug 31:2023.08.29.555423. doi: 10.1101/2023.08.29.555423. bioRxiv. 2023. Update in: Cell Rep Med. 2024 Feb 20;5(2):101417. doi: 10.1016/j.xcrm.2024.101417. PMID: 37693377 Free PMC article. Updated. Preprint.
-
Cell Fusion Induced by a Fusion-Active Form of Human Cytomegalovirus Glycoprotein B (gB) Is Inhibited by Antibodies Directed at Antigenic Domain 5 in the Ectodomain of gB.J Virol. 2020 Aug 31;94(18):e01276-20. doi: 10.1128/JVI.01276-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641474 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Interventions for the prevention and treatment of herpes simplex virus in patients being treated for cancer.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD006706. doi: 10.1002/14651858.CD006706.pub2. Cochrane Database Syst Rev. 2009. PMID: 19160295
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
