This is a preprint.
Regulatory Plasticity and Metabolic Trade-offs Drive Adaptive Evolution of Alternative Flagellar Configurations in Pseudomonas aeruginosa
- PMID: 40766384
- PMCID: PMC12324275
- DOI: 10.1101/2025.07.29.667523
Regulatory Plasticity and Metabolic Trade-offs Drive Adaptive Evolution of Alternative Flagellar Configurations in Pseudomonas aeruginosa
Abstract
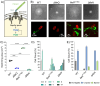
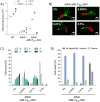
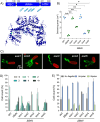
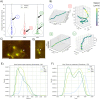
Evolutionary constraints governing flagellar number in bacterial pathogens remain poorly understood. While related Pseudomonas species are hyperflagellated, P. aeruginosa maintains strict monoflagellation through the FleQ-FleN regulatory circuit. Here, we demonstrate that FleN dosage is essential for maintaining monoflagellation and bacterial fitness. Wild-type P. aeruginosa consistently displayed unipolar monoflagellation, while ΔfleN mutants developed over two-to-five flagella per cell in uni- or bipolar arrangements. Hyperflagellated ΔfleN cells exhibited severe fitness defects including reduced growth rates, attenuated virulence in nematode infection models, and competitive disadvantages in co-culture experiments. Remarkably, ΔfleN cells rapidly evolved suppressor mutations in fleQ that partially restored growth and motility without always restoring monoflagellation. Five independent suppressor alleles mapped to critical FleQ functional domains (four in the AAA+ ATPase domain, one in the DNA-binding domain), suggesting reduced protein activity that rebalances the disrupted regulatory circuit. Single-cell motility analysis revealed that suppressor strains exhibit heterogeneous swimming dynamics, with subpopulations achieving wild-type speeds despite carrying multiple flagella. Proteomic analysis demonstrated that hyperflagellation triggers extensive cellular reprogramming beyond flagellar components, affecting metabolic pathways, stress responses, and signaling networks. While hyperflagellated cells suffered complete loss of pathogenicity in animal infection models, environmental selection under viscous conditions could drive wild-type cells to evolve enhanced motility through specific fleN mutations. These findings suggest that bacterial flagellar regulatory circuits function as evolutionary capacitors, normally constraining phenotypic variation but enabling rapid adaptation to alternative motility configurations when environmental pressures exceed the performance limits of standard monotrichous flagellation.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Characterization of Flagellum and Toxin Phase Variation in Clostridioides difficile Ribotype 012 Isolates.J Bacteriol. 2018 Jun 25;200(14):e00056-18. doi: 10.1128/JB.00056-18. Print 2018 Jul 15. J Bacteriol. 2018. PMID: 29735765 Free PMC article.
-
Direct and indirect pathways linking the Lon protease to motility behaviors in the pathogen Pseudomonas aeruginosa.PLoS Pathog. 2025 Jun 25;21(6):e1013288. doi: 10.1371/journal.ppat.1013288. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40561152 Free PMC article.
-
Rescue of bacterial motility using two- and three-species FliC chimeras.J Bacteriol. 2025 Aug 11:e0051724. doi: 10.1128/jb.00517-24. Online ahead of print. J Bacteriol. 2025. PMID: 40788109
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Sexual conflict drive in the rapid evolution of new gametogenesis genes.Semin Cell Dev Biol. 2024 Jun-Jul;159-160:27-37. doi: 10.1016/j.semcdb.2024.01.005. Epub 2024 Feb 2. Semin Cell Dev Biol. 2024. PMID: 38309142 Review.
References
-
- Allan D. B., Caswell T., Keim N. C., van der Wel C. M., & Verweij R. W. (2024). soft-matter/trackpy: 0.6.2rc1. Zenodo. 10.5281/zenodo.10674547 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
