This is a preprint.
CD45 sequestration lowers the signaling threshold in lymphocytes and enhances anti-tumor immunity
- PMID: 40766423
- PMCID: PMC12324387
- DOI: 10.1101/2025.07.29.667400
CD45 sequestration lowers the signaling threshold in lymphocytes and enhances anti-tumor immunity
Abstract
CD45 plays a central role in immune signal regulation by controlling the spatial dynamics of phosphatase activity through steric segregation of its bulky rigid extracellular domain. To modulate CD45 activity, here we develop and characterize protein engineering approaches to induce multivalent clustering of CD45, effectively mimicking the endogenous local receptor sequestration during immune synapse formation. In doing so, we engineer a biologic that enables precise, tunable control over CD45 surface localization and activity. CD45 sequestration exhibited striking synergy when administered in combination with intratumorally anchored IL-12 therapy, markedly delaying tumor progression and extending survival in syngeneic murine melanoma and carcinoma models. Immune profiling revealed that CD8+ T cells are essential mediators of this synergistic antitumor response. Mechanistically, IL-12 initiates a wave of antigen generation and T cell priming, while CD45 sequestration subsequently enhances tumor-specific CD8+ T cell activation, expansion, and functional states within the tumor-draining lymph node. These findings suggest that CD45 sequestration lowers the activation threshold of T cells, broadens the tumor-reactive T cell repertoire, and therefore promotes more robust tumor-specific T cell responses. Altogether, we establish CD45 as a promising novel target for cancer immunotherapy, capable of potentiating strong anticancer immune responses.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures
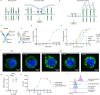
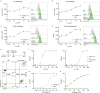

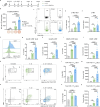

References
-
- Hermiston M. L., Xu Z. & Weiss A. CD45: A critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 21, 107–137 (2003). - PubMed
-
- Davis S. J. & Merwe P. A. van der. The kinetic-segregation model: TCR triggering and beyond. Nat. Immunol. 7, 803–809 (2006). - PubMed
-
- Merwe P. A. van der, Davis S. J., Shaw A. S. & Dustin M. L. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 12, 5–21 (2000). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
