This is a preprint.
Cat brains age like humans: Translating Time shows pet cats live to be natural models for human aging
- PMID: 40766475
- PMCID: PMC12324510
- DOI: 10.1101/2025.07.31.667772
Cat brains age like humans: Translating Time shows pet cats live to be natural models for human aging
Abstract
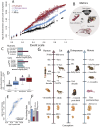
Translating biological time across species is a powerful tool to identify new models of human aging and disease. Currently, it is not clear whether any animal reaches an age comparable to a human in their 80s. Most species seem to age differently compared with humans. Some preliminary observations suggest that cats may share common patterns of aging with humans. Cats could serve as a promising model for human aging. Here, we find corresponding ages between cats, humans and other species to test whether cats can live to the equivalent of a human in their 80s. We analyzed 3,754 observations across species from sudden and gradual changes in anatomy, physiology, and behavior. Some of these data are from clinical records, whereas others are from brain scans using high-resolution MRI (7T and 3T). We studied pet cats, research colony cats, and wildcats living in zoos to encapsulate species variation in the speed of development and aging. We found that cat and human brains atrophy with age, and that their age-related patterns in brain aging are sufficiently similar that we could use them to generate cross-species age alignments. We also found that human postnatal development is stretched compared with cats and mice. Interestingly, some pet cats that visit clinics are much older than those in colonies. Therefore, cats, and especially pet cats, are natural model systems of human aging. Our findings call for increased integration across veterinary and human medicine to understand aging.
Figures







References
-
- Berg L, McKeel DW, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL (1998) Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of neurology. 55:326–35. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
