This is a preprint.
Hemoglobin alpha regulates T-lymphocyte activation and mitochondrial function
- PMID: 40766506
- PMCID: PMC12324427
- DOI: 10.1101/2025.08.01.668160
Hemoglobin alpha regulates T-lymphocyte activation and mitochondrial function
Abstract
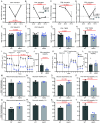
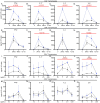
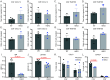
We have recently discovered hemoglobin alpha a1 (Hbα-a1 mRNA and Hbα protein) in T-lymphocytes and previously reported that its expression was sensitive to mitochondrial redox perturbations. However, outside of its occurrence and basic characterization, the functional role of Hbα in T-lymphocytes remained unknown. Herein, we identify Hbα in both CD4+ and CD8+ T-lymphocyte subsets, and found its expression is highly dynamic, differs between the two subtypes, and is dependent upon activation stage. Further, the loss of Hbα by use of a novel T-lymphocyte-specific Hbα knock-out mouse impairs mitochondrial function, dysregulates cytokine production, and lowers the activation threshold primarily in CD4+ T-lymphocytes, indicating a critical role for Hbα within this subset. While these data suggested the loss of Hbα in T-lymphocytes may promote aberrant activation of autoreactive T-lymphocytes, surprisingly, we discovered that mice lacking Hbα in T-lymphocytes exhibited reduced severity of experimental autoimmune encephalomyelitis (EAE) compared to wild-type control animals. Interestingly, T-lymphocytes lacking Hbα in vivo appeared to function identically to wild-type controls, which did not explain the protection against EAE. In contrast, T-lymphocyte Hbα knock-out mice displayed significantly reduced levels of circulating immunoglobulins and CD40L expression compared to their wild-type counterparts during EAE, suggesting possible impaired intercellular communication. These data elucidate a previously unrecognized role for Hbα in T-lymphocyte function, which may have implications for hemoglobin-related diseases (i.e., hemoglobinopathies).
Keywords: EAE; Hemoglobinopathy; Immune; Inflammation; redox.
Conflict of interest statement
Conflict of Interest Statement: The authors have declared that no conflict of interest exists.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
