This is a preprint.
A multimodal approach for visualization and identification of electrophysiological cell types in vivo
- PMID: 40766549
- PMCID: PMC12324179
- DOI: 10.1101/2025.07.24.666654
A multimodal approach for visualization and identification of electrophysiological cell types in vivo
Abstract
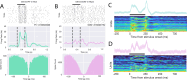
Neurons of different types perform diverse computations and coordinate their activity during sensation, perception, and action. While electrophysiological recordings can measure the activity of many neurons simultaneously, identifying cell types during these experiments remains difficult. To identify cell types, we developed PhysMAP, a framework that weighs multiple electrophysiological modalities simultaneously to obtain interpretable multimodal representations. We apply PhysMAP to seven datasets and demonstrate that these multimodal representations are better aligned with known transcriptomically-defined cell types than any single modality alone. We then show that such alignment allows PhysMAP to better identify putative cell types in the absence of ground truth. We also demonstrate how annotated datasets can be used to infer multiple cell types simultaneously in unannotated datasets and show that the properties of inferred types are consistent with the known properties of these cell types. Finally, we provide a first-of-its-kind demonstration of how PhysMAP can help understand how multiple cell types interact to drive circuit dynamics. Collectively, these results demonstrate that multimodal representations from PhysMAP enable the study of multiple cell types simultaneously, thus providing insight into neural circuit dynamics.
Keywords: Cell types; Dimensionality Reduction; Electrophysiology; Multi-Modal Analysis; optotagging.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures







References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
