This is a preprint.
Modulation of the 14-3-3σ/C-RAF "auto"inhibited complex by molecular glues
- PMID: 40766587
- PMCID: PMC12324506
- DOI: 10.1101/2025.07.30.667769
Modulation of the 14-3-3σ/C-RAF "auto"inhibited complex by molecular glues
Abstract
Molecular glues, compounds that bind cooperatively at protein-protein interfaces are revolutionizing chemical biology and drug discovery, allowing the modulation of traditional "undruggable" targets. Here, we focus on the native protein-protein interaction (PPI) of C-RAF, a key component of the MAPK signaling pathway, with the scaffolding protein 14-3-3. Although extensive drug discovery efforts have focused on the MAPK pathway, its central role in oncology and developmental disorders (RASopathies), still requires alternative approaches, moving beyond direct kinase inhibition. Indeed, stabilization of native PPIs is a relatively unexplored territory in this pathway. The function of C-RAF is regulated on multiple levels including dimerization, phosphorylation and complex formation with the hub protein 14-3-3. 14-3-3 prevents C-RAF activation by molecular recognition and binding at the phospho-serine 259. We used a fragment-merging approach to design a molecular glue scaffold that would bind to the composite surface of the 14-3-3/C-RAF "auto"inhibited complex. The synthesized molecular glues stabilized the 14-3-3/C-RAF complex up to 300-fold in biophysical assays; their glue-based mechanism of action was confirmed with several crystal structures of ternary complexes. Selectivity among the other RAF isoforms and other RAF phosphorylation sites was evaluated with biophysical assays. The best compounds showed excellent selectivity among a broad panel of 80 14-3-3 clients. Validation in cell assays showed on-target engagement, enhanced phosphorylation levels of the C-RAF pS259 site, reduced RAF dimerization and reduced ERK phosphorylation. Overall, this approach enables chemical biology studies on a C-RAF site that is intrinsically disordered prior to 14-3-3 binding and has not been targeted previously. These molecular glues will be useful as chemical probes and starting points for further drug discovery efforts to elucidate the effect of native PPI stabilization in the MAPK pathway with applications in oncology and RASopathies.
Keywords: 14-3-3; C-RAF; PPI; covalent; fragment merging; induced proximity; molecular glue; stabilizer.
Conflict of interest statement
The authors declare the following competing interest: M.R.A., C.O., and L.B. are founders of Ambagon Therapeutics.
Figures

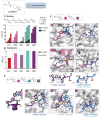

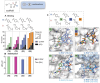

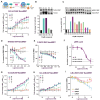
References
-
- Davies H.; Bignell G. R.; Cox C.; Stephens P.; Edkins S.; Clegg S.; Teague J.; Woffendin H.; Garnett M. J.; Bottomley W.; Davis N.; Dicks E.; Ewing R.; Floyd Y.; Gray K.; Hall S.; Hawes R.; Hughes J.; Kosmidou V.; Menzies A.; Mould C.; Parker A.; Stevens C.; Watt S.; Hooper S.; Wilson R.; Jayatilake H.; Gusterson B. A.; Cooper C.; Shipley J.; Hargrave D.; Pritchard-Jones K.; Maitland N.; Chenevix-Trench G.; Riggins G. J.; Bigner D. D.; Palmieri G.; Cossu A.; Flanagan A.; Nicholson A.; Ho J. W. C.; Leung S. Y.; Yuen S. T.; Weber B. L.; Seigler H. F.; Darrow T. L.; Paterson H.; Marais R.; Marshall C. J.; Wooster R.; Stratton M. R.; Futreal P. A. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417 (6892), 949–954. 10.1038/nature00766. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
