This is a preprint.
SkeletAge: Transcriptomics-based Aging Clock Identifies 26 New Targets in Skeletal Muscle Aging
- PMID: 40766611
- PMCID: PMC12324255
- DOI: 10.1101/2025.07.28.667277
SkeletAge: Transcriptomics-based Aging Clock Identifies 26 New Targets in Skeletal Muscle Aging
Abstract
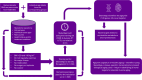
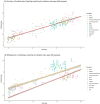
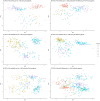
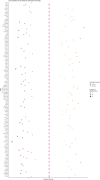
Identifying the set of genes that regulate baseline healthy aging - aging that is not confounded by illness - is critical to understating aging biology. Machine learning-based age-estimators (such as epigenetic clocks) offer a robust method for capturing biomarkers that strongly correlate with age. In principle, we can use these estimators to find novel targets for aging research, which can then be used for developing drugs that can extend the healthspan. However, methylation-based clocks do not provide direct mechanistic insight into aging, limiting their utility for drug discovery. Here, we describe a method for building tissue-specific bulk RNA-seq-based age-estimators that can be used to identify the ageprint. The ageprint is a set of genes that drive baseline healthy aging in a tissue-specific, developmentally-linked fashion. Using our age estimator, SkeletAge, we narrowed down the ageprint of human skeletal muscles to 128 genes, of which 26 genes have never been studied in the context of aging or aging-associated phenotypes. The ageprint of skeletal muscles can be linked to known phenotypes of skeletal muscle aging and development, which further supports our hypothesis that the ageprint genes drive (healthy) aging along the growth-development-aging axis, which is separate from (biological) aging that takes place due to illness or stochastic damage. Lastly, we show that using our method, we can find druggable targets for aging research and use the ageprint to accurately assess the effect of therapeutic interventions, which can further accelerate the discovery of longevity-enhancing drugs.
Keywords: Ageprint; Biological Aging; Epigenetic Clock; Longevity Drug Discovery; Skeletal Muscle.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
High social status males experience accelerated epigenetic aging in wild baboons.Elife. 2021 Apr 6;10:e66128. doi: 10.7554/eLife.66128. Elife. 2021. PMID: 33821798 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Ahn H., Kim D. W., Ko Y., Ha J., Shin Y. B., Lee J., Sung Y. S., & Kim K. W. (2021). Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia. Ageing Research Reviews, 70, 101398. 10.1016/j.arr.2021.101398 - DOI - PubMed
-
- Avelar R. A., Ortega J. G., Tacutu R., Tyler E. J., Bennett D., Binetti P., Budovsky A., Chatsirisupachai K., Johnson E., Murray A., Shields S., Tejada-Martinez D., Thornton D., Fraifeld V. E., Bishop C. L., & de Magalhães J. P. (2020). A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biology, 21(1), 91. 10.1186/s13059-020-01990-9 - DOI - PMC - PubMed
-
- Bell C. G., Lowe R., Adams P. D., Baccarelli A. A., Beck S., Bell J. T., Christensen B. C., Gladyshev V. N., Heijmans B. T., Horvath S., Ideker T., Issa J.-P. J., Kelsey K. T., Marioni R. E., Reik W., Relton C. L., Schalkwyk L. C., Teschendorff A. E., Wagner W., … Rakyan V. K. (2019). DNA methylation aging clocks: Challenges and recommendations. Genome Biology, 20(1), 249. 10.1186/s13059-019-1824-y - DOI - PMC - PubMed
-
- Belsky D. W., Caspi A., Houts R., Cohen H. J., Corcoran D. L., Danese A., Harrington H., Israel S., Levine M. E., Schaefer J. D., Sugden K., Williams B., Yashin A. I., Poulton R., & Moffitt T. E. (2015). Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences of the United States of America, 112(30), E4104–4110. 10.1073/pnas.1506264112 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
