This is a preprint.
Metabolic and behavioral effects of neurofibromin result from differential recruitment of MAPK and mTOR signaling
- PMID: 40766628
- PMCID: PMC12324403
- DOI: 10.1101/2025.07.25.666841
Metabolic and behavioral effects of neurofibromin result from differential recruitment of MAPK and mTOR signaling
Abstract
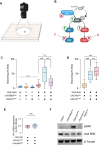
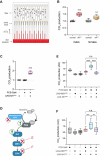
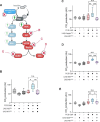
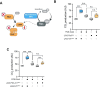
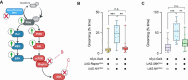
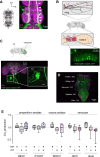
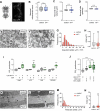
Neurofibromatosis type 1 results from mutations in the Neurofibromin 1 gene and its encoded neurofibromin protein. This condition produces multiple symptoms, including tumors, behavioral alterations, and metabolic changes. Molecularly, neurofibromin mutations affect Ras activity, influencing multiple downstream signaling pathways, including MAPK (Raf/MEK/ERK) and PI3K/Akt/mTOR signaling. This pleiotropy raises the question of which pathways could be targeted to treat the disease symptoms, and whether different phenotypes driven by neurofibromin mutations exhibit similar or diverging dependence on the signaling pathways downstream of Ras. To test this, we examined metabolic and behavioral alterations in the genetically tractable Drosophila neurofibromatosis type 1 model. In vivo genetic analysis revealed that behavioral effects of neurofibromin were mediated by MEK signaling, with no necessity for Akt. In contrast, metabolic effects of neurofibromin were mediated by coordinated actions of MEK/ERK and Akt/mTOR/S6K/4E-BP signaling. At the systemic level, neurofibromin dysregulated metabolism via molecular effects of Nf1 in interneurons and muscle. These changes were accompanied by altered muscle mitochondria morphology, with no concomitant changes in neuronal ultrastructure or neuronal mitochondria. Overall, this suggests that neurofibromin mutations affect multiple signaling cascades downstream of Ras, which differentially affect metabolic and behavioral neurofibromatosis type 1 phenotypes.
Conflict of interest statement
Competing interest statement The authors declare no competing interests.
Figures

References
-
- Constantino JN, Zhang Y, Holzhauer K, Sant S, Long K, Vallorani A, et al. Distribution and Within-Family Specificity of Quantitative Autistic Traits in Patients with Neurofibromatosis Type I. The Journal of pediatrics. 2015;167(3):621–6 e1. Epub 20150604. doi: 10.1016/j.jpeds.2015.04.075. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
